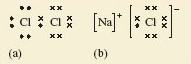2.4 Sketching the Lewis structures for Cl2 and NaCl
The description of the bonding in Cl2 molecules and the ionic solid NaCl, in the previous sections took a lot of words.
However there is a way of doing this, very concisely, using simple diagrams, the chemical symbols you met earlier and “dots and crosses” to represent valence electrons.
Here’s how it works.
Figure 3(a) below shows the Lewis structure for the Cl2 molecule. The valence electrons are grouped in pairs, to reflect the pairing of electrons you’ve previously seen for atomic orbitals.
The shared pair is shown as a dot and a cross in the centre. The remaining electrons in the valance shell of each atom are distributed in pairs, so taking the shared electrons into account there are eight electrons around each chlorine atom in the molecule. Note that the use of a dot or a cross is just to indicate where a particular electron originates, they are purely symbols. There’s no difference between an electron represented by a cross and one allocated a dot.
Likewise the ions in sodium chloride have also been represented in this way in Figure 3(b).
The chloride anion has eight outer electrons; recall the chlorine atom has gained an electron to complete its octet. Similarly sodium loses its outer electron to form a cation, so the Lewis structure of the cation has no electrons shown.
In both structures, the formation of a chemical bond involves the production of a new electron pair in the outer shell of chlorine. However, in Cl2, because the two atoms are identical, the electron pair must be equally shared between the two atoms. In contrast you can view the electron pair as being located on the chloride anion in NaCl.
You will return to this point later, but first a look at a molecule which does not follow the octet rule.