3.1 The shapes of some simple fluorides.
In this section you will be looking at the geometries adopted by some fluorides of several elements.
The examples you will be looking at are: BeF2, BF3, CF4, PF5, SF6 and IF7; their shapes, which have been experimentally determined, are shown in Figure 7.
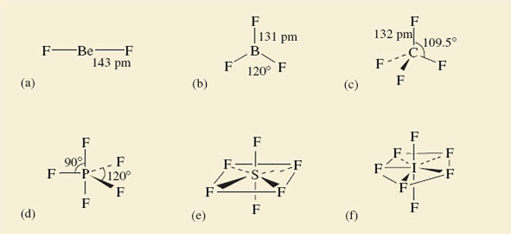
Beryllium difluoride (BeF2) is a glassy non-molecular solid at room temperature, but the BeF2 molecule (Figure 7a) is obtained when the solid is vaporised by heating it to 1 200 °C. It is linear; that is, the sequence of atoms F—Be—F lies on a straight line. The spatial arrangement of the neighbouring atoms around a particular atom is said to be the coordination of that atom.
At 25 °C, BF3, CF4, PF5, SF6 and IF7 are all gases containing molecules with the shapes shown in Figure 7b-f.
In BF3, all four atoms lie in the same plane, the boron atom forming three B—F bonds to three fluorine atoms at the corners of an equilateral triangle This arrangement of fluorines around boron is called trigonal planar.
In CF4 there is tetrahedral coordination of the four fluorine atoms around carbon. This is the situation which you have already met in the methane molecule.
The coordination in PF5, SF6 and IF7 is a little more complicated and perhaps best described by starting with the horizontal planes containing the central atom of these molecules.
In PF5, this plane contains three P—F bonds directed towards the corners of an equilateral triangle as in BF3; in SF6, it contains the sulfur atom with four surrounding fluorines at the corners of a square.
What does the horizontal plane contain in IF7?
The iodine atom, and five I—F bonds directed towards five fluorine atoms at the corners of a regular pentagon.
In all three cases, the coordination is then completed by two other bonds to fluorine at 90° to those in the horizontal plane, one pointing up, and the other pointing down. These arrangements in PF5, SF6 and IF7 are called trigonal bipyramidal, octahedral and pentagonal bipyramidal, respectively.
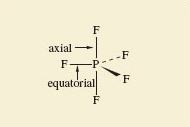
In the octahedral molecule SF6, all the fluorine atoms are equivalent. From each of the fluorine atoms, the “view of the rest of the molecule” looks the same. But in PF5 and IF7, this is not so. There are two kinds of fluorine position: equatorial positions in the horizontal plane, and axial positions at right-angles to it. In Figure 8, these two kinds of position are labelled for the trigonal-bipyramidal arrangement in PF5.
All this begs a rather obvious question, why do molecules adopt the shapes they do? This will be addressed in the next section.