4 Sodium D-lines and the hydrogen atom
Energy levels are demonstrated in the context of a sodium lamp in Video 3. Watch it now and then do Activity 4.

Transcript: Video 3 Sodium D-lines.
Activity 4 Energy levels
a.
zero
b.
1
c.
2
d.
5
e.
10
The correct answer is d.
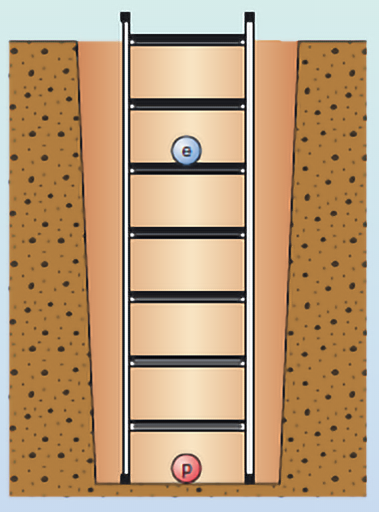
Video 3 introduced the concept of energy levels. These can be thought of as the rungs on a ladder (Figure 5).
Figure 5 represents the simplest atom, hydrogen. This atom has one electron ‘in orbit’ around the nucleus (labelled as the blue circle with the letter ‘e’). The nucleus of a hydrogen atom contains just one proton (labelled as the red circle with the letter ‘p’). The rungs on the ladder represent discrete and separate energy levels. In the ‘ground state’, the electron would be on the first rung of the ladder. This state is given the value n = 1. (Please note that this is not the same ‘n’ as in the diffraction equation). If you give the electron exactly the right amount of energy, it will then move up to the next rung of the ladder. Here it would be the second rung where n = 2. You can test your understanding of this in the next activity.
Activity 5 Energy levels in a hydrogen atom (ladder analogy)
a.
n = 5
b.
n = 4
c.
n = 3
d.
n = 2
e.
n = 1
The correct answer is a.
a.
n = 3
b.
n = 4
c.
n = 5
d.
n = 6
e.
n = 7
The correct answer is e.
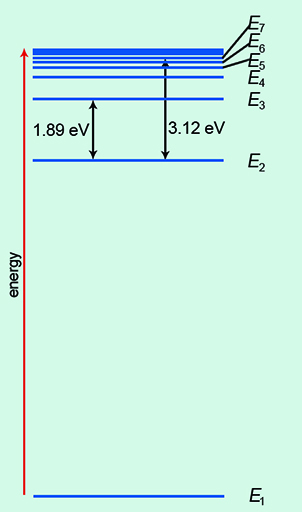
Of course, the real picture is more complicated than a simple ladder! In Figure 6, the ground state of energy is labelled E1. As you move up the energy levels, they bunch closer together. The electrons can transition, or move, between each energy level. Indeed, there are numerous permutations.
You can see that the energy units of eV, or electronvolts, are used here. At this size in the quantum world, the usual unit of energy, the joule, is much too big.
Now complete this section on energy levels by doing Activity 6.
Activity 6 Energy levels in a hydrogen atom
a.
7
b.
6
c.
5
d.
3
e.
4
The correct answer is a.
a.
E1 and E2
b.
E1 and E3
c.
E2 and E3
d.
E3 and E4
e.
E4 and E5
The correct answer is c.
a.
E1 and E7
b.
E2 and E7
c.
E3 and E7
d.
E4 and E7
e.
E5 and E7
The correct answer is b.
You will now look at quantum energy levels.