CLN3 disease
CLN3 disease (also known as Batten Disease) is frequently referred to as a juvenile dementia. This is due to the devastating series of neurological symptoms caused by this disease, which begins to show symptoms in childhood and progresses through the teenage years into early adulthood. The disease is fatal, and individuals with CLN3 disease typically only live until around their late 20s (Schulz et al., 2013). CLN3 disease is caused by the inheritance of a mutant copy of the CLN3 gene from both parents, which causes a protein found inside cells to be incorrectly assembled, affecting cellular function (Butz et al., 2020).
The symptoms start around 4-8 years of age with eyesight deterioration. Affected children also begin to suffer seizures, along with changes to mood and behaviour and a decline in speech and motor skills causing increasing difficulties with movement; similar to some forms of dementia in the elderly. However, in addition to the striking dementia-like symptoms, CLN3 patients also begin to suffer heart problems towards the end of their lives.
How does a healthy heart work and what makes it beat?
Your heart is a fascinating muscle: It keeps contracting regularly throughout your lifetime, on average 2 billion times, without a rest. This organ ensures that blood is pumped efficiently through the body to provide it with oxygen, whilst removing waste products. To do this, two circular routes of blood flow start from the heart: blood with a high oxygen content leaves the left ventricle via the aorta and passes through the body, where oxygen is passed on to tissues and organs such as muscles and the brain. It returns to the heart via the right atrium and ventricle with a depleted oxygen content. In the second circuit, the blood then is pumped to the lungs, where the oxygen content is replenished, and blood with a high oxygen content returns to the left atria and ventricle to start the circuit again.
The main chambers, which have a thick muscular wall to pump the blood efficiently to the lungs or the body are called ventricles, with the left ventricle being the thickest of all the heart chambers as it is responsible for pumping blood all around the body. Blood returns into the heart via two smaller, less muscular, chambers called atria, which pass the blood to the ventricles. Valves at the openings through which blood enters the chambers prevents it from flowing in the reverse direction.
The resting heart rate varies between individuals. During exercise or in response to stress, the heart rate increases. A condition where an individual has a chronically low heart rate is called bradycardia, which can be a sign of potentially serious illness. Conversely, an elevated resting heart rate is known as tachycardia which can also be dangerous to health, as it could mean the heart does not fill up with enough blood before contracting.
The normal heart rhythm is controlled by an electrical signal, triggered by a small set of cells called the sinoatrial node; which set in motion the signal that passes through the heart via the atrioventricular node. This signal is responsible for triggering events in the cell, such as the release of calcium ions, which triggers the contraction. A healthy heart will respond promptly and synchronously to the signal which allows the chambers to be emptied of blood (Bers, 2002, Eisner et al., 2017).
What does a heart look like (under a microscope)?
A heart actually only vaguely resembles the heart shape that we all think about. When looking at it in detail under a microscope, it is fascinating to see how well organised it is to allow the co-ordinated contractions to happen. The contractile cells are specialised muscle cells called cardiac myocytes. The image of heart tissue from a mouse shows the structure of a cardiac myocyte, taken at extremely high resolution, using an electron microscope. (Figure 1). The myocyte is made up of z-lines which are equally spaced and all of the myofibres are aligned, indicating that the muscle could carry out a uniform contraction in one direction. The dark structures between the fibres are mitochondria, which are the organelles providing energy to cells. Because muscles need a lot of energy, they contain many mitochondria which form an interconnected network
How can a heart break?
You heard that too low or too high a heart rate can be detrimental to the function of the heart. When this is the case, tissues - including the heart itself - do not receive enough oxygen to function effectively. This can lead to disruptions in the conduction of the electrical signal as well as the potential for cardiac myocyte death. Another frequent cause of heart problems are structural changes, for example the formation of scar tissue, which can form in the place of cardiac myocytes which had died. These scars can also interrupt the electrical signal and can alter the normal synchronous heartbeat. Taken together, such changes disrupt the ability to efficiently pump blood, and perform it’s important function necessary to sustain our life.
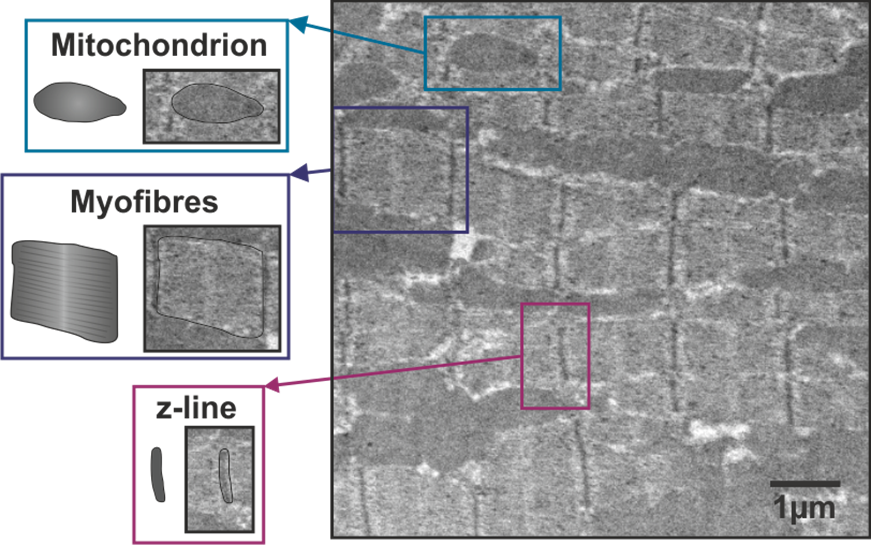 Electron micrograph of a ventricular cardiac myocyte from a mouse
Electron micrograph of a ventricular cardiac myocyte from a mouse
How is the effect of CLN3 on heart function studied?
The majority of laboratory research into this disease has focussed on investigating why patients suffer vision loss and neurological symptoms; making the eyes and especially the brain most commonly studied. CLN3 research is mainly carried out by clinicians who work directly with the affected individuals and record their progression, and by laboratory research scientists who work with models of cells or animals containing the CLN3 mutation.
In laboratory research, animals carrying the CLN3 genetic mutation have been developed and are compared to healthy animals. Again, very few of these studies have looked at the effects of the heart, but some have recorded observations such as an enlarged heart in the CLN3 mouse and a large, unusually shaped heart in zebrafish carrying the CLN3 mutation (Wager et al., 2016, Staropoli et al., 2012). Preliminary work using electron microscopy (as pictured) has also suggested a difference in the structure of the heart muscle in the CLN3 mouse model (Rietdorf et al., 2019).
Why is it important to understand the heart problems in CLN3 disease if they happen so late in life?
As the life span of those with CLN3 disease starts to gradually extend, due to gradual improvements in diagnosis and care, there is an increased interest in the heart problems that arise later in life. While the ultimate goal is to cure the disease and allow the affected individuals to lead a normal life, any improvement to length and quality of life is of great importance. This is reflected in the recent advances in research focussing on treatment options for diseases such as CLN3 (Cooper and Mole, 2020). A greater understanding of exactly why the heart suffers in this illness, will open doors to better therapeutic intervention to help these affected individuals to live healthier and longer lives.
Acknowledgments: Dr Daniel Johnson for his consultation on the writing of this article. Dr Igor Kraev and Dr Radka Gromnicova for their expertise and support with electron microscopy. NCL Foundation for their support, especially through the ‘Young Investigators’ meetings and their focus on bringing together the CLN3 research community.


Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews