Resource 6: Ideas for demonstrations
![]() Background information / subject knowledge for teacher
Background information / subject knowledge for teacher
Expansion of a solid: ball and ring
When both the ball and ring are at room temperature, the ball can be dropped through the ring. Heating the ball makes the metal expand, so it cannot pass through the ring. As the ball cools down, it contracts and will fit through the ring again.
Key words: solid, heating, cooling, expansion, expand, contraction, contracts particles, vibration, vibrate, energy

Expansion of a liquid: model thermometer
Fill a boiling tube with coloured water, then insert a narrow glass tube (inserted through a cork or bung) into the neck of the boiling tube: make sure the end of the glass tube is in the water. As you heat the water in the boiling tube, you should see the column of coloured liquid in the glass tubing get higher and higher, because the liquid is expanding as it is heated. This is how liquid-in-glass thermometers work.
Key words: liquid, heat expansion, expands, particles, movement, energy
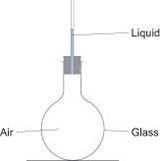
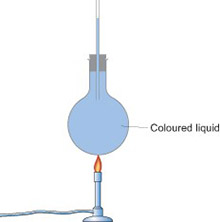
Expansion of a gas: liquid in a tube/bubbling flasks
You can show this by using a test tube or boiling tube with a piece of capillary tubing inserted into it through a bung. The capillary tubing should have a very small amount of water in it. If you warm the tube with your hands, you should see the water rise up the tube: it is being pushed up by the air which is trapped inside the test tube.
Another way to do this is to use a boiling tube or round bottomed flask with a narrow glass tube inserted into it. Clamp the tube or flask so that the open end of the glass tubing is below the surface of a trough of water. When you warm the air in the flask with your hands, bubbles of air will come out of the tubing into the water.
You can demonstrate the opposite process – contraction of a gas as it cools – if you have an empty plastic drinks bottle with a screw top. Pour some hot water into the bottle, swirl it round, then pour it out again. Screw the top back on straight away. Leave the bottle to cool down and watch as it collapses (because the cooling air inside contracts).
Key words: gas, heating expansion, expands, contracts, contraction, particles, collisions, energy
Dissolving and diffusion
Potassium permanganate crystals in water
Get a glass trough, or a large glass beaker or glass bowl and put water in it to about 10 cm depth. The container must be on a steady surface, and give a good view of the contents either from the side or from above. It helps to have some white paper under the container and behind it, so it will be easier to see any colour changes in the water. Let the water settle completely, then drop one or two (no more) potassium permanganate crystals into the water. The colour spreads out slowly from the crystal and the crystal gets smaller as it dissolves in the water. Purple colour is evidence that there is some potassium permanganate in that bit of the water. If left long enough, the purple colour will spread throughout all of the liquid and the colour will be the same intensity, instead of being deepest near the crystal. The slow colour spreading is evidence of diffusion.
Key words: potassium permanganate crystal, solid, dissolving, dissolves, diffusion, particles, collisions, random
Perfume in air
Spray some strong perfume in one corner of the room. Ask your students to put their hand up when they can smell the perfume. The perfume particles will diffuse through the room, with the people nearest to where it was sprayed, smelling it first. Comparison with the potassium permanganate experiment shows that diffusion is faster in gases than in liquids.
Key words: diffusion, particles, collisions, spaces, random
Resource 5: Students’ writing



