5.2.3 Coagulation and flocculation
After aeration, coagulation takes place, to remove the fine particles (less than 1 µm in size) that are suspended in the water. In this process, a chemical called a coagulant (with a positive electrical charge) is added to the water, and this neutralises the negative electrical charge of the fine particles. The addition of the coagulant takes place in a rapid mix tank where the coagulant is rapidly dispersed by a high-speed impeller (Figure 5.5).
Since their charges are now neutralised, the fine particles come together, forming soft, fluffy particles called ‘flocs’. (Before the coagulation stage, the particles all have a similar electrical charge and repel each other, rather like the north or south poles of two magnets.) Two coagulants commonly used in the treatment of water are aluminium sulphate and ferric chloride.
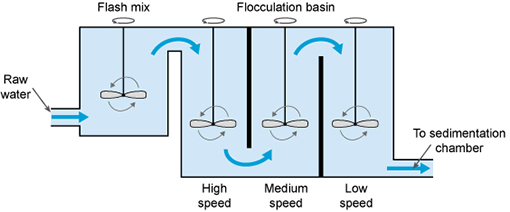
The next step is flocculation. Here the water is gently stirred by paddles in a flocculation basin (Figure 5.5) and the flocs come into contact with each other to form larger flocs.
The flocculation basin often has a number of compartments with decreasing mixing speeds as the water advances through the basin (Figure 5.6(a)). This compartmentalised chamber allows increasingly large flocs to form without being broken apart by the mixing blades. Chemicals called flocculants can be added to enhance the process. Organic polymers called polyelectrolytes can be used as flocculants.
5.2.2 Aeration
