5.2.7 Supplementary treatment
Supplementary treatment may sometimes be needed for the benefit of the population. One such instance is the fluoridation of water, where fluoride is added to water. It has been stated by the World Health Organization that ‘fluoridation of water supplies, where possible, is the most effective public health measure for the prevention of dental decay’ (WHO, 2001). The optimum level of fluoride is said to be around 1 mg per litre of water (1 mg l–1).
On the other hand, as you learned in Study Session 2, in the Rift Valley of Ethiopia, the water resources contain a higher concentration of fluoride than is desirable. Tekle-Haimanot et al. (1995) found that the level of fluoride in drinking water from deep wells there ranged from 1.5 to 36 mg l–1. The safe level for fluoride is 1.5 mg l–1.
What does excess fluoride in the water lead to?
As mentioned in Study Session 2, in children it can cause mottling of teeth and prolonged exposure can cause skeletal fluorosis and crippling.
In such high-fluoride areas, removal or reduction of fluoride (termed defluoridation) is essential. The simplest way of doing this is to blend the high-fluoride water with water that has no (or very little) fluoride so that the final mixture is safe. If this is not possible, technical solutions may be applied. Two of these, the Nakuru Method and the Nalgonda Technique, used in Ethiopia, are described below.
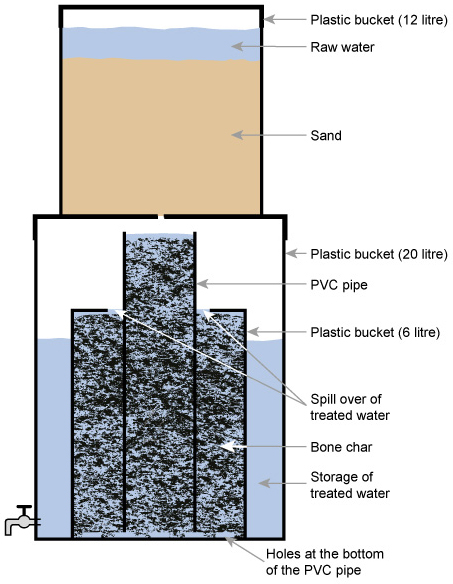
The Nakuru Method (Figure 5.8) involves a filter with bone char (charcoal produced from animal bone) and calcium phosphate to adsorb the fluoride (Kung, 2011). There have been reservations on the use of bone char, and alternatives for defluoridation, such as activated alumina, are being tested in Addis Ababa (Alemseged, 2015).
The Nalgonda technique for defluoridation (Suneetha et al., 2008) uses aluminium sulphate and calcium oxide to remove fluoride. The two chemicals are added to and rapidly mixed with the fluoride-contaminated water and then the water is stirred gently. Flocs of aluminium hydroxide form and these remove the fluoride by adsorption and ion exchange. The flocs are then removed by sedimentation.
5.2.6 Chlorination
