10.2.3 Disinfection
Disinfection is the final stage of water treatment in the home. All water treated using one or more of the previous steps will need to be disinfected to ensure that all pathogens are killed. The three common methods of disinfection are described below.
Boiling
Boiling drinking water is a simple way of killing pathogens and is suitable for use at household level. Boiling destroys pathogens such as bacteria and viruses, and any parasite ova (eggs) present in water. The water should be heated until large bubbles come continuously to the surface of the water (referred to as a ‘rolling boil’), for at least 5 minutes. This has been shown to inactivate cholera and Shigella organisms (Luff and Clarke, 2006). If the location of the site is at a high elevation (as in Addis Ababa) the water should be boiled for longer. Boiling will make the water taste flat but this can be remedied by shaking the water in a clean bottle, or by adding a pinch of salt to one litre of water. If the water is turbid with particles, it should be filtered before boiling. Water should be boiled, cooled and stored, all in the one container and consumed within 24 hours to prevent re-contamination.
Can you think of disadvantages associated with boiling?
Fuel has to be obtained, and scalding accidents can occur if people are careless. But it is still the simplest way of ensuring that water is safe to drink.
Solar disinfection (SODIS)
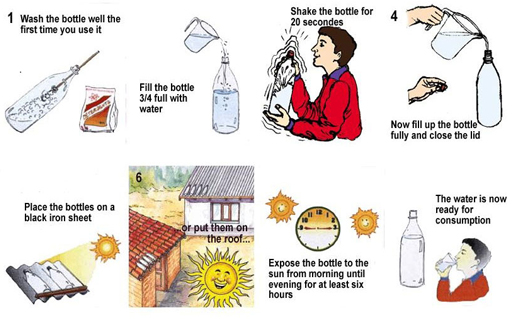
Solar disinfection, known as SODIS, is a water treatment method that uses ultraviolet (UV) radiation and high temperature from the sun to destroy micro-organisms in water. The technique requires only some clear plastic bottles (without labels) and sunlight (Figure 10.8).

The steps to take for SODIS are shown in Figure 10.9. In Step 5, the bottles are placed on a corrugated iron sheet (often used as roofing material) which is painted black so that it retains heat from the sun, speeding up the rate of heating of the water.
Chemical disinfection using chlorine
Chlorine solution, also known as sodium hypochlorite or bleach, is the most affordable and widely available chemical for household water treatment. Typically the procedure is to add a capful of chlorine solution to water in a 20–25 litre storage container, then stir and wait for 30 minutes. As you learned in Study Session 5, this period of time is referred to as the contact time. After this, the water can be used.
Chlorination is effective if the water is not turbid. If the water is turbid, micro-organisms can shelter within the particles and escape the effect of the chlorine. Solids should therefore first be removed by sedimentation or filtration. It is important that some residual chlorine remains in the water at the time it is used, so that it stays safe to drink. A minimum concentration of 0.5 mg l–1 is recommended; this will kill any organism that enters the water later.
10.2.2 Filtration
