2.1 Ethical considerations
A key part of research is ensuring that participants have consented to be involved in your research. There are two fundamental reasons for obtaining written consent using a consent form. Firstly, the consent form provides a structure around which researchers can explain to participants the key aspects of the research that involves them. This helps to make sure that participants give ‘informed consent’, so they understand:
- key aspects of the research
- participation is voluntary
- the focus and purpose of the study
- what to expect from participating
- whether they will receive an incentive for taking part
- that they can pause the interview, stop the interview, or withdraw from the study
- how their data will be used, managed, and stored.
Secondly, the consent form acts as documentary evidence that participants have received information about, and have understood, these key aspects of the project. The form acts as a mutual agreement about the roles and responsibilities of interviewer and interviewee or researcher and participant, protecting the researcher as well as the participant.
The first stage in this process is ensuring that participants are clearly informed about the study they are signing up to. If they don’t understand the research, they cannot consent to be involved in it. A typical way of doing this is to provide potential participants with what we collectively call ‘participant materials’. These should be shared as part of the recruitment process, so potential participants can consider them before deciding to enrol in the research. Participant materials may include an information leaflet about the study and a copy of the consent form.
Once participants have read the materials you have provided, they need a way to indicate that they consent to be enrolled into the study. This can be done in several ways – through a physical document that participants sign, an electronic document which is emailed and which they sign electronically, or through an internet form, usually supplied as a link.
For the Reproductive Bodylore project, we created several participant documents. Participant Materials include a ‘Friends & Family Information Sheet’ and a ‘Friends & Family Online Consent Form’. The information sheet was a word document, which was emailed to potential participants. The consent form was an online survey link which was emailed to potential participants.
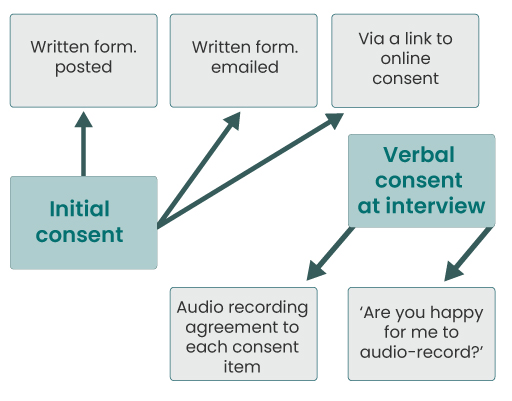
It is good practice to use a two-stage consent process. This means that interviewees give some form of written consent (as outlined above) and that you also discuss consent at the beginning of the interview. Sometimes, this might be done by reading out each of the consent items to participants and obtaining their informed consent. In other cases it may simply be asking a question, or questions, checking they have given consent. Figure 2 shows the two-stage consent process, and some ways you might check consent at each stage.
Having signed a consent form and given verbal consent at the beginning of an interview does not mean participants cannot withdraw from the study. They should be free to leave the interview at any point, and to withdraw their data up until the point when analysis has begun.