3.3 Fertilisers
For most of you, the food you eat also relies on the oil (and gas) industry. In the developed world, our diets rely on cheap food transported significant distances. How far food has been transported depends hugely on the choices we make – whether it’s the intensively reared meat flown in from the other side of the world, or the organic farm produce driven from just a few tens of miles away, both require transport, and currently most of that transport relies on oil-based fuels.
It isn’t just transport from farm to consumer either – since the beginning of the mechanisation of agriculture (using petroleum-powered tractors), a significant amount of oil-based fuels are used during the farming and processing of crops, meat, fish and related products.
Beyond just moving food crops around, fertilisers are (currently) produced using natural gas from the same geological reservoirs as liquid oil (more on that later). Something like 50% of all global food production relies on nitrogen-based fertilisers.
Nitrogen (N) is critical to plant growth, mainly because it is an essential component of amino acids, which go on to form proteins. Although nitrogen makes up 78% of the atmosphere, getting it into a form which can be taken up into plants is tricky. For that to happen, it has to be ‘fixed’ into a form such as anhydrous ammonium nitrate (NH4NO3) or urea (CO(NH2)2).
Enter Fritz Haber, who, along with Carl Bosch developed the Haber–Bosch process to mass produce fixed nitrogen in the form of ammonia (NH3), from which many nitrogen-based fertilisers are made.
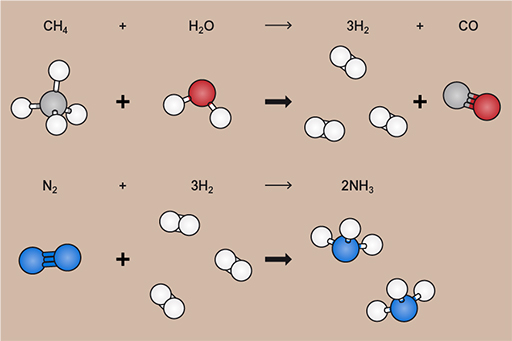
The Haber–Bosch process itself takes nitrogen and hydrogen, under high pressure and temperature and in the presence of a sequence of catalysts, and converts them into ammonia. The nitrogen can be taken from the air, but the hydrogen comes from breaking down methane (CH4) into hydrogen (H2) and carbon monoxide (CO) using water in a process called steam reforming.
The methane currently comes from natural gas (predominantly, it can also be sourced from coal, but that’s less common now), which, as you’ll see later this week, comes from the same sort of geological formations as the liquid oil.