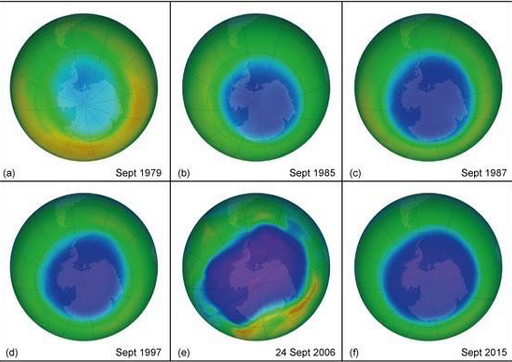
3.2 Ozone loss
You saw in Session 3 that ozone (O3) is among the greenhouse gases in our atmosphere. As well as absorbing infrared radiation, ozone absorbs most of the Sun’s ultraviolet radiation, shielding us from its harmful effects such as skin cancer. Ozone depletion by human use of chlorofluorocarbons (CFCs) was a major environmental issue of the late 1980s until the Montreal Protocol restricted the use of ozone-depleting substances. The increasing size of the ‘ozone hole’ and recent signs of recovery can be seen in Figure 6.
CFCs deplete the ozone layer through a cycle of reactions started by light. When CFCs are broken down by UV radiation from the Sun, this produces highly reactive chlorine atoms that react with ozone and destroy it. This is a very destructive process and a single chlorine atom can continue to deplete ozone until it reacts with something different, such as nitrogen oxides.
Sulfate aerosols pose a risk to the ozone layer because they react with nitrogen oxides. This reduces one mechanism of removing chlorine atoms, the result is the amount of chlorine increases, and therefore ozone depletion increases.
One small silver lining is that this risk should become less important. The 1987 Montreal Protocol has been effective in reducing chlorine in the atmosphere from CFCs, so the sulfates will eventually no longer be able to amplify their effect on the ozone layer (Keith, 2013, p. 68).
