2.1 Experiment 3: Ice tray experiment
Solids, liquids and gases have very different properties that you will already be aware of on a practical level. For example:
- solids can typically be held and keep their shape
- liquids can flow, changing their shape to fill the space provided but without changing their volume
- gases are often invisible and change their shape and volume to fill whatever space is available.
The reason these states of the same material differ is to do with the behaviour of the particles (atoms and/or molecules) from which they are comprised:
- Particles in solids are packed together closely, held by strong forces called bonds. The particles can vibrate but they cannot move around freely, so a solid holds its shape.
- Particles in liquids are arranged randomly and are still held together by bonds, but the bonds are weak enough to allow the particles to move around each other.
- Particles in gases are further apart and are not held together by bonds. This allows the gas particles to move freely in all directions, expanding to fill the space they occupy.
Under special conditions, things can exist in all three states at the same time. Mostly though, only one or two states are seen at the same time. The most obvious example is for water, which, at standard pressure is a liquid at room temperature but a solid (ice) at temperatures below 0 °C, and a gas (steam) above 100 °C.
This week’s experiment is all about the physical properties of different household liquids and how they change when they are frozen. You will also investigate whether or not the frozen liquid sinks or floats when placed into its own liquid form at room temperature.
Many of you will have performed part of this experiment already, perhaps adding an ice cube or two to a glass of water to cool it down. What happens when you add these ice cubes to your glass of water? Does the ice cube sink or does it float?
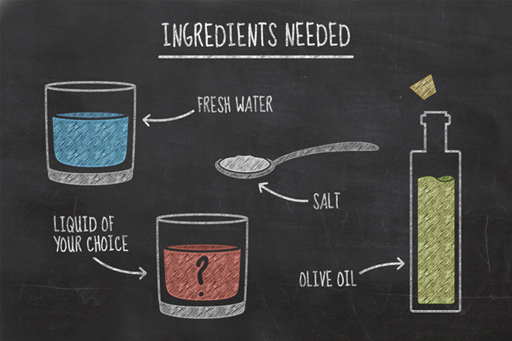
To conduct this experiment, you will need:
- an ice cube tray
- fresh water
- salt water (approximately 2 tablespoons of salt added to 500 ml of water)
- olive oil
- another liquid of your choice – be creative here, but avoid substances which might be hazardous!
- four glasses
- your activity booklet [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)]
- a freezer.
Before beginning an experiment scientists often use their previous experiences or preconceptions to hypothesise about the results of an experiment. For example, we know that ice floats on water, so a scientist might then hypothesise that the solid state of all the other substances will also float on their liquid counterparts.
You will notice that we have only specified three liquids (water, salt water and olive oil); however, you should choose a fourth liquid yourself. This is a chance for you to be creative. Look around your home and see what you can find.
What other liquid are you going to test? And what hypothesis can you make? Did you use any previous experiences or preconceptions to make this hypothesis? Post your ideas below. Scientists are curious folk with lots of ideas and interests, so explore your inner scientist.
