1.5.2 Osmosis explained
You’ve seen the results of your experiment and it should be clear that water is somehow moving in and out of your cucumbers – this process is known as osmosis.
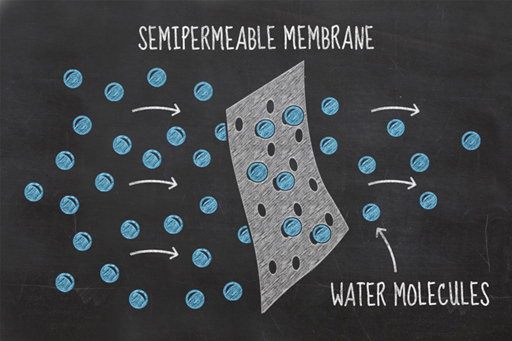
Imagine two solutions of different concentrations, divided by a partition which allows small particles through it, but not large particles. This type of boundary is known as a partially permeable membrane. In this environment, the process of osmosis will occur spontaneously because the concentrations of water molecules on either side of the partition, or membrane, naturally try and equalise.
In your experiment, the water in the glass and the fluid inside the cucumber’s cells are separated by the cucumber’s cell walls which are partially permeable membranes. Salt cannot pass through these membranes, but water can. By adding salt to the water, you made its salt concentration higher and therefore lowered the concentration of water in the mixture. This gives the cucumber cells a relatively higher water concentration than that in the glass. The water in the cucumber cells tries to equalise these different concentrations by moving from the cells to the saltwater solution. As a result, the cucumber loses water and becomes a bit squishy. This environment is referred to as hypertonic.
In your other glass, containing the distilled water, the opposite effect was seen. Water flowed from the pure water (a higher concentration region) into the cucumber cells (which have a lower concentration of water). In this hypotonic environment, the water tries to equalise by moving into the cucumber cells, inflating them, and causing the cells to become firm. This is known as turgor, and it is the turgor pressure in plant cells that keeps them rigid. Without it, plants wilt and their cellular functions will begin to decline.
When the concentrations on either side of the membrane are equal, the condition is known as isotonic, and water moves randomly from one side of the membrane to the other, but with no pressure gradient to drive it, the rate is the same in both directions.
