1.6 SI units
SI units (from the French, Système International d’Unités) are the internationally agreed units of measurement used in most countries, and in the different branches of science.
The system arose during the French Revolution of the 1790s with the kilogram and the metre, but was not completed until the 1960s. Standard units of measurement were agreed to describe things like weights and distances, based on multiples of ten. Before this, people measured things in many different ways and this caused confusion and mistakes. For example, the ways of measuring length were most commonly based on things which come to hand; there were hands, spans, feet, inches, yards, chains, furlongs, miles, leagues, fathoms, cables, links and rods. While these measurements are easy to use at one level, combining them can be haphazard: for example, most people know there are 12 inches in a foot, but how many know how many feet there are in a chain?
Length
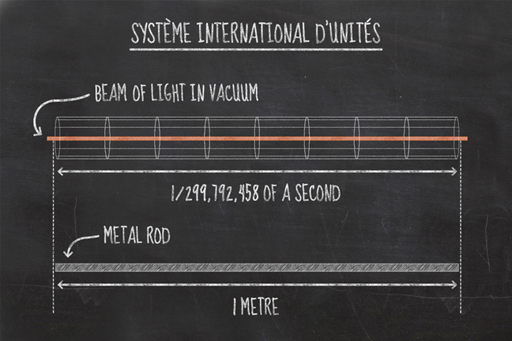
For length, the SI unit was chosen to be the metre. For many years a metal rod was used as the base for all measurements of the metre, but more recently such measurements have been based on natural phenomena. The metre is defined relative to the speed of light. A beam of light travels 299,792,458 metres in one second, so a metre is the distance that light travels in 1/299,792,458 of a second. But why change to this definition? Why would a metal rod not be good enough? The main issue is that all metals expand and contract depending on temperature so if we use a real metal rod as the definition of a metre, then a metre at room temperature would be different to a metre on an icy morning.
The SI system has seven base units from which all other units can be derived. To make units bigger or smaller, rather than coming up with a different name, as with inches, feet and yards, a prefix is added to the base unit. The prefix ‘centi’ means one hundredth, so a centimetre is one hundredth of a metre. ‘Milli’ means one thousandth, so a millimetre is one thousandth of a metre. A thousand metres are known as a kilometre, as ‘kilo’ comes from a Greek word meaning a thousand. So we have millimetres, centimetres, metres and kilometres, and this same approach can be applied to other units, for example, milligrams, grams, kilograms etc.
Time
The base unit of time is the second. It is defined by the vibrations of an atom of caesium. In fact, a second is defined as 9,192,631,770 vibrations of a caesium-133 atom. The vibrational rates of atoms are extremely regular. This has led to atomic clocks which keep time to an accuracy of 1 second in 300 million years. You might think that that is needlessly accurate, but this accuracy has everyday applications in telecommunications: GPS clocks need to be this accurate in order to measure a position with precision, while many applications of the internet require high-accuracy time standards.
Mass
For mass, the system is slightly odd, as it’s the only one to already include a Greek prefix in the name of the base unit, the kilogram. It is also the only one still defined by a physical object; the International Prototype Kilogram, a metal cylinder stored in a vault in Paris, and all means of measuring an object’s mass can be traced back to this.
Temperature
Temperature uses a unit called the kelvin, named after the British scientist, Lord Kelvin. In everyday life we generally use Celsius (or as it is sometimes known, centigrade). The Celsius scale is defined with 0 °C (degrees Celsius) and 100 °C as the temperatures where water changes its state between ice, water and steam. The kelvin scale has units of the same size, but uses the coldest possible temperature as its lower limit. Heat is the measure of the motion of atoms in a substance. At zero on the kelvin scale, all motion in atoms ceases, it’s not possible to be any colder. This is referred to as absolute zero. This temperature corresponds to about -273 °C (to the nearest degree). So 0 °C is equivalent to 273 K. The coldest places in the universe reach as low as 2.7 K, nowhere is absolute zero.
Remember that units are important descriptors. Five kilograms isn’t the same as 5 metres, or 5 kelvin, so always add units if you’re describing natural phenomena.