1.2.2 Alpha, beta and gamma radiation
The imbalance of forces within unstable nuclei leads to the emission of particles.
Here you’ll find out about three types of particle that can be emitted; α, β and γ or alpha, beta and gamma particles. Collectively they are often referred to as radiation from a radioactive substance.
Alpha particles
Alpha particles are the largest of the emitted particles and are positively charged. They are composed of two protons and two neutrons that are bonded together, which is the same as a helium-4 nucleus. This makes them the most massive of the types of radioactive emission.
The nucleus therefore loses two protons and becomes a different element!
For example, if emits an α particle it will become ; uranium has transformed into thorium.
Alpha particles are easier to stop than other forms of radiation because they are bigger than the other forms and because they have a double positive charge (remember that a proton has a single positive charge). They can be stopped by a sheet of paper or by clothing. This is important because they cause a lot of damage if they interact with biological tissue. Even when they are travelling in air, their positive charge means that they attract electrons and quickly become nothing more than harmless helium.
Beta particles
Beta particles are negatively charged and turned out to be an electron ejected from a nucleus. They have a much lower mass than alpha particles. In neutral atoms, electrons exist outside the nucleus, and have a negative charge equal and opposite in magnitude to the positive charge on a proton.
In β-decay, a neutron inside a nucleus changes into a proton and emits an electron, that is the β-particle (another particle called an antineutrino is also emitted but it has no charge and almost no mass, and can be ignored in the present context).
In this case the initial nucleus has gained a proton and so again a new element may be formed. Note that the mass number doesn’t change as the number of nucleons is the same. The number of neutrons has reduced by one, and the number of protons has increased by one.
For example, if emits a β particle it will become ; caesium has transformed into barium.
A β-particle has more kinetic energy than a normal electron and carries a single negative charge. It’s harder to stop than an α-particle and can get through paper or clothing but is stopped by denser materials such as water or aluminium. Once they are stopped, the β-particles simply become part of the material they are in, like any other electron.
Gamma particles
Gamma rays are high-energy electromagnetic radiation emitted by radioactive elements. They possess energy but no mass. The electromagnetic spectrum, shown in Figure 5, is the range of all possible energies of electromagnetic radiation. A photon can be defined as the basic unit, or elementary particle, of electromagnetic radiation. Like visible light, a γ-ray is just energy but a γ-photon has more energy than a photon of visible light or even of X-rays.
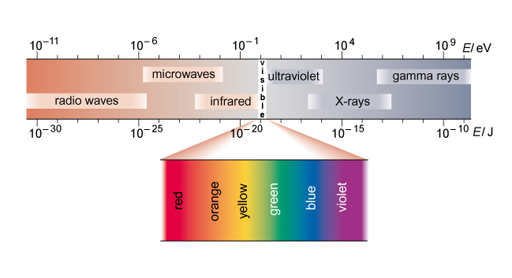
Unlike α or β radiation, loss of a γ particle does not change the composition of the nucleus, although it does lose energy. Gamma emission usually occurs together with α- or β-emission and it is rare to get gamma rays emitted on their own.
Because they have no mass or charge and high energy, γ-rays are more difficult to stop than β- or α-particles and it takes dense materials such as lead, or concrete, to absorb their energy and stop them. This is an issue you’ll consider in Week 2 when you’ll hear about the storage of radioactive waste.
All three types of radiation can damage human cells and this damage can lead to cancers developing in the area affected. All three types of radiation can be detected by a Geiger counter.
Next, you will find out where radiation is found.
