3.2.4 Exclusion zone
The meltdowns within the reactors led to some of the radioactive fission products leaking into the environment.
This was exacerbated by release of gases and material by the explosions and the venting of gases that occurred to try to reduce pressure within the reactors and limit further explosions. In addition, the entire plant was flooded with seawater that provided a medium to transport the radioactive isotopes into the surrounding area and into the sea!
From 17 March, the decision was made to drop seawater from helicopters on the reactors targeting reactors 3 and 4 in particular. This would create radioactive steam that also contaminated the environment, but it was getting desperately necessary to cool the reactors down.
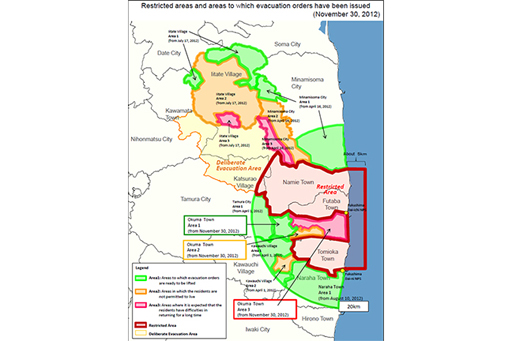
In response to the radiation leaks the Japanese government ordered an evacuation around the Daiichi plant initially of 2km but as the crisis developed it extended up to 20km by the evening of 12 March. The exclusion zone around the site is shown in Figure 7. In total about 160,000 people were evacuated as a result of the meltdowns.
Clean-up of the exclusion zone could not begin in earnest until December 2011 when the nuclear plant was cool enough to be deemed in ‘cold shutdown’.
Two particular isotopes were of concern, both fission products spread extensively within the exclusion zone:
- Iodine-131. This is commonly ingested by humans as an element within many foodstuffs. Iodine-131 is a beta emitter with a half-life of eight days and can pose a serious health risk. This means that is active initially, but will decay rapidly enough to cease to be a threat in the long term. Iodine tablets were distributed to workers and those near the plant. The tablets are taken so that this non-radioactive iodine will be taken by the body to the thyroid gland. The aim is to ‘fill up’ the thyroid gland so that any radioactive iodine ingested will pass through the body and not linger in the thyroid gland to cause damage.
- Caesium-137. This is also readily incorporated into the human body and its salts are water soluble. It is also a beta emitter and has a much longer half-life of 30 years. This means that caesium-137 continues to be a threat for many years.
The clean-up involved removal of the contaminated topsoil within the exclusion zone. This is fraught with many difficulties including that there is a variable radiation within the zone, that each worker is only allowed a limited time within the zone and the issue of where to store the radioactive soil once collected. Another problem is the huge area that needs to be covered. It is estimated that the clean-up will cost tens of billions of pounds.
In the next section, you will look at the measurement of radioactivity within the soil four months after the crisis.