4.1.1 Carbon emissions and global warming
There is much research going into energy resources that have low carbon emissions because it’s recognised that carbon dioxide damages the Earth’s atmosphere.
Most of the Earth’s atmosphere is composed of nitrogen and oxygen but there are other gases in the atmosphere that concern us here: water and carbon dioxide.
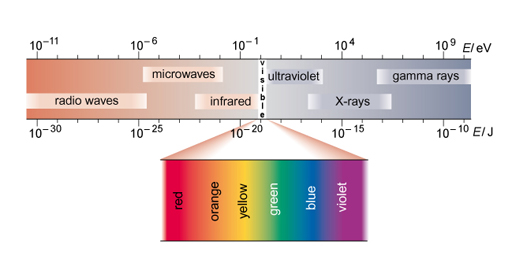
These gases consist of molecules which are made up of more than one type of atom and as a consequence of this the bonds between the atoms vibrate. These vibrations enable the gases to absorb infrared (IR) radiation which comes from the Earth’s surface. If you look at Figure 3 you can see that IR is next to visible light, and this part of the electromagnetic spectrum has slightly lower energy.
It means that IR cannot pass through the gases, it is absorbed and re-emitted by the gases in the atmosphere, warming up the Earth’s surface. This warming is called the greenhouse effect and gases such as water and carbon dioxide that are able to absorb IR are called greenhouse gases. If the amount of greenhouse gas in the atmosphere increases, then more energy is absorbed by the atmosphere and re-emitted towards Earth.
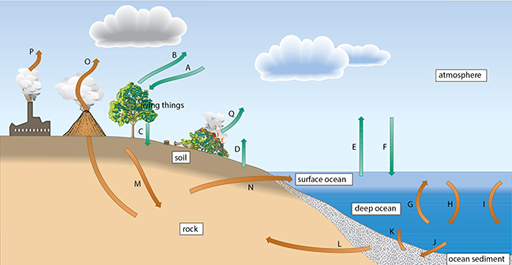
Carbon exists in many different forms on the Earth and the carbon cycle consists of the flow of carbon between different reservoirs – these are shown schematically in Figure 4. There are many natural processes that exchange carbon between the different reservoirs.
One of these reservoirs is the atmosphere. If more carbon dioxide enters the atmosphere, it is possible that more will be retained within it and there will be an increased greenhouse effect and temperature rise leading to global warming.
There are processes that remove carbon from the atmosphere – for example, photosynthesis into the reservoir of ‘living things’ – and throughout Earth’s history the flow between reservoirs has been able to adjust in times of increased temperature or cooling. However, burning fossil fuels over the past 100 years has led to a new and additional process by which carbon can be transferred to the atmosphere.
When fossil fuels burn in air, oxygen reacts with organic carbon to form carbon dioxide and water vapour, usually released into the atmosphere. These are both greenhouse gases. The fear is that our prolonged burning of fossil fuels in power stations and vehicles may lead to a situation where the carbon cycle is unable to adjust and carbon (as carbon dioxide) will build up in the atmosphere and lead to irreversible climate change.
Consequently, there have been moves recently to reduce the release of carbon dioxide to a sustainable level – one that would allow the carbon cycle to cope with its absorption from the atmosphere.
In both the UN Kyoto protocol and at a G8 summit, steps have been taken to legally require nations to limit their carbon dioxide emissions. The UK is committed to reducing its carbon emissions to 80% of its 1990 value by 2050. You can have a look at the UK regulations on the Committee on Climate Change [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] website (n.d.). You may want to find the requirements of your own country.
As a result of these issues everyone is encouraged to reduce their carbon footprint as individuals. You can calculate your carbon footprint in the next section.
