4.2.2 What is nuclear fusion?
You will recall from Week 1 that the origin of the energy that is released is the change in mass and binding energy when a large nucleus splits into two lighter ones.
This works for massive atoms such as uranium, plutonium or thorium. You looked at the fission reaction:
The mass of the fission products and the three neutrons is less than of the U-236, although the number of nucleons is the same and this missing mass is released as energy due to equivalence of mass expressed by the equation E = mc2.
We can also consider this in terms of binding energy – the energy released is due to the difference in binding energy of the nuclei involved.
Nuclear fusion
Let us look at a fusion reaction:
Here hydrogen is fused with deuterium – the heavier isotope of hydrogen that contains one neutron to produce helium-3.
As with the fission reaction above, the number of nucleons is the same on each side but the mass of the helium nucleus is less than that of the hydrogen plus deuterium. This missing mass is converted to energy and released. In fact, the mass difference tends to be larger than with fission so that more energy is released in fusion reactions.
You may well be puzzling over how both splitting nuclei apart and fusing them together can produce energy. The full answer to this is beyond the scope of this course, but suffice to say that the physics within nuclei mean that the following is true.
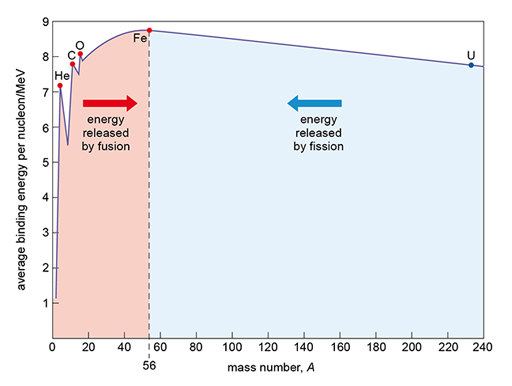
- heavy nuclei with more than 56 nucleons release energy by undergoing fission
- light nuclei with fewer than 56 nucleons release energy by undergoing fusion.
This is shown in Figure 12.
The advantages of fusion over fission
Nuclear fusion is often heralded as the ultimate future technology although this has been the case for some time! It is nonetheless pursued as it has significant advantages over fission.
- The fuel for fusion is hydrogen. Hydrogen is by far the most common element in the Universe and is plentiful on Earth. Water contains hydrogen, for example. The fuels used are often the isotopes of hydrogen called deuterium (hydrogen-2) and tritium (hydrogen-3). Deuterium can be extracted from water and tritium can be produced from lithium in the Earth’s core. Both of these resources are plentiful and will last for millions of years.
- Unlike the radioactive products of fission, fusion produces no long-lived isotopes. Only plant components become radioactive and these will be safe to recycle or dispose of conventionally within 100 years.
- The process of fusion produces a huge amount of energy and only very tiny amounts of fuel need to be used. This means that nuclear incidents, such as those you heard about last week, are not possible with fusion.
- As with fission, there are no carbon emissions. The only by-products of fusion reactions are small amounts of helium, which is an inert gas that will not add to atmospheric pollution.
In the next section, you will consider the difficulties of achieving fusion.