1.1 Chemical elements
The basic form of matter, containing only atoms of the same atomic number, and which cannot be chemically broken down into simpler substances is a chemical element.
Chemists have given each element a name and a symbol. This is the same as that given to the atoms it contains. As you will see on working through this module, some elements have an upper-case letter as their chemical symbol (e.g. hydrogen, H) and others have an upper-case letter followed by a lowercase letter (e.g. helium He).
Consider the simplest element hydrogen, this has been given the symbol H.
So the H represents both the element hydrogen and the atoms of hydrogen.
Taking this a step further, hydrogen has an atomic number of one indicating there is one proton in the nucleus, and one electron in orbit around it.
The atomic number of the element lithium is 3, How many protons are in the nucleus, and how many electrons are orbiting the nucleus?
Answer
There are 3 protons, and given the atom is an uncharged (neutral) species this charge must be balanced by 3 electrons.
The elements may solids, liquids or gases at room temperature, and some examples, together with their chemical symbols are shown in Figure 2.
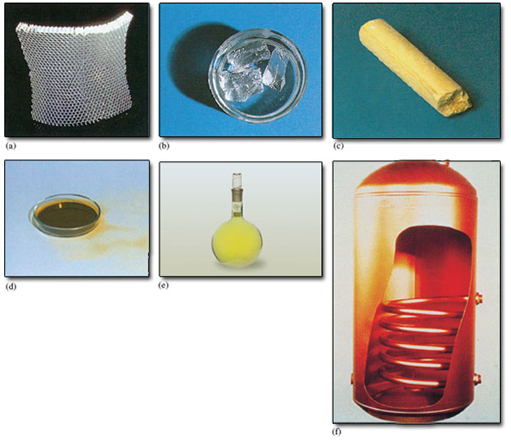
What is the state of matter of each of the elements shown in Figure 1.2?
Answer
Aluminium, sodium, sulfur and copper are solids. Bromine is a liquid and chlorine is a gas.
So at this point you have a picture of the basic structure of an atom, however there is one further subatomic particle to consider. This will be introduced in the next section.
