2.1 Some further examples of molecular substances
The element carbon forms a vast range of compounds, often in combination with hydrogen, nitrogen, oxygen and other elements such as sulfur and phosphorus.
This whole area of the subject is referred to as organic chemistry.
Organic compounds are almost entirely molecular, common examples are methane, glucose, ethanol and vinegar (acetic acid).
This point is illustrated in Figure 4, which shows the grouping of the atoms in the molecules of two important organic compounds.
Figure 4a shows the structure of aspirin, the best-known painkiller, and a precautionary treatment of heart conditions. The molecule in Figure 4b is RDX, a military high explosive.
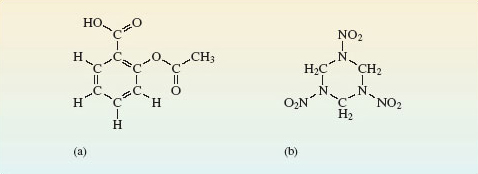
Don’t worry about the names used for organic compounds either here or elsewhere in this course. Where such compounds are discussed, you will only be looking at differences in the structure of their molecules; just view the names as labels.
Let’s now return to where we started and look in a bit more detail at compounds that can be described as non-molecular.
