1.1 Valency and the chemical bond
Up to this point you have simply treated valencies as just been numbers that are used to predict the formulae of compounds. But in the case of covalent substances they can tell us so much more.
In particular, they reveal how the atoms are linked together in a molecule. This information is obtained from a two-dimensional drawing of the structural formula of the molecule. (Note that structural formulae cannot be assumed to carry any implications about molecular shape.)
Consider, for example, the molecules H2, Cl2, NCl3 and CH4. Their structural formulas are shown here as Structures 4.1-4.4. They can be drawn correctly by ensuring that the number of lines or bonds emerging from any atom is equal to its valency.
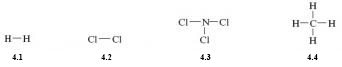
H2 and Cl2 are simply represented as a single line between the two atoms, both hydrogen and chlorine have a valency of one.
Recalling that nitrogen has a valency three, and chlorine a valency of one; NCl3 is shown with three bonds emerging from the nitrogen atom and one from each chlorine atom.
Account for the structure of methane CH4 (4.4) in terms of the valency of its atoms.
Carbon has a valency of four, so will form four bonds to hydrogen, which has a valency of one.
At this point, check that you are comfortable with these bonding ideas by attempting the following.
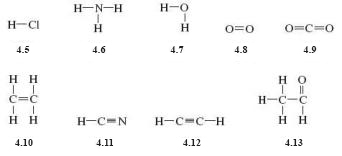
Draw structural formulas for the following molecular substances: hydrogen chloride (HCl), ammonia (NH3), water (H2O), oxygen (O2), carbon dioxide (CO2), ethane (C2H4), hydrogen cyanide (HCN), ethyne (C2H2) and ethanal (CH3CHO).
The structural formulae are as shown below: