1.7 Dative bonds
So far, the bonds in Lewis structures have been shared electron pairs made by taking one electron from each of the two bound atoms.
But this need not necessarily be the case.
To take an example, if you mix the colourless gases ammonia, NH3 and boron trifluoride BF3, a reaction occurs. This is shown in the following video.
Describe the appearance of the product of the reaction of NH3 and BF3.
The product appears as a dense white smoke.
Now let’s try to explain this reaction using the Lewis structures of the reacting molecules.
As you have seen already, ammonia (NH3) comprises three electron pair bonds to hydrogen atoms and one non-bonded pair being allocated to nitrogen (Structure 4.14). And in the last section you also saw that the Lewis structure of boron trifluoride left the boron atom two electrons short of a noble gas configuration Structure 4.21.
The Lewis structures of these two molecules are reproduced below.
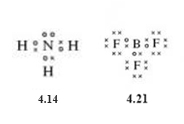
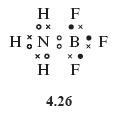
If an electron-pair bond is created by allowing the non-bonded electron pair on nitrogen in the ammonia molecule to be shared between the nitrogen and boron atoms, the Lewis structure (Structure 4.26) can be written in which all atoms, including boron, have a noble gas shell structure.
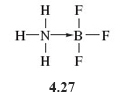
A bond in which the electron pair is provided by just one of the bonded atoms, as is the case here, is called a dative bond (or coordinate bond).
Note that, in order to focus attention on the bonding electrons, the non-bonded electron pairs on the fluorine atoms have been omitted from Structure 4.26 - this is common practice.
In order to differentiate dative bonds from the more familiar bonds in which each bound atom contributes an electron to the electron pair, dative bonds are represented as arrows running from the ‘donor’ atom (in this case nitrogen) to the 'receptor' atom (in this case boron) (4.27).