3 When Lewis structures don’t work
Lewis structures provide a convenient, relatively straightforward and by and large successful of representing the bonding in molecules.
But they don’t always tell the whole story.
Consider the oxygen molecule (O2).
Sketch a Lewis structure for oxygen (O2)
This is shown below (4.38).
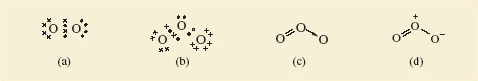
If you take the Lewis structure of the oxygen molecule at face value, then it looks like all the bonding electrons are arranged in pairs, and there is a double bond between the two oxygen atoms. However, the following video suggests otherwise.
Note: the term paramagnetism won’t be discussed in detail here, suffice to say this is a weak form of magnetism as opposed to the effect you experience in magnetic materials made of iron, or rare-earth elements for example.
What were your observations of the behaviour of liquid oxygen in a magnetic field?
Liquid oxygen was attracted to the poles of the magnet (in contrast to liquid nitrogen which passed straight through).
Without going into details here, the implication of the above result is that the oxygen molecule contains one or more unpaired electrons.
The Lewis structure indicates all electrons are paired, so clearly is not an adequate representation of the bonding in O2.
So although they are very useful (in fact later in this course you will see how drawing a Lewis structure is the basis for predicting the shape of molecules) and routinely used, chemists often have to use more sophisticated theories to provide a more detailed and refined picture of the bonding in molecules.
In the next session you will start to look at the principles underpinning reactions and the reactivity of substances.
