1 Molecular shape affects molecular reactivity
To start your exploration of the effect of the shape of molecules on how they react, let us start with two simple examples: methane, CH4, and bromomethane, CH3Br.
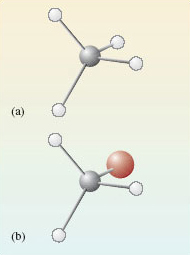
In both cases, the carbon atoms form four single bonds and it turns out that these are directed towards the corners of a tetrahedron (you will see why this is later).
The resulting molecular shapes are shown in Figure 2 as ball-and-stick representations. The grey sphere is the carbon atom, the white spheres represent the hydrogen atoms and the red sphere is bromine.
If you take bromomethane (Figure 2b) and successively replace each hydrogen atom by a methyl group, CH3, what are the formulas of the resulting molecules?
- CH3CH2Br, (CH3)2CHBr and (CH3)3CBr.
Ball-and-stick representations of each of these molecules are shown at the top of Figure 3. Again at each carbon atom, there are four bonds directed towards the corners of a tetrahedron, and the complexity of the molecular shape increases from left to right, in line with an increase in the number of carbon atoms.
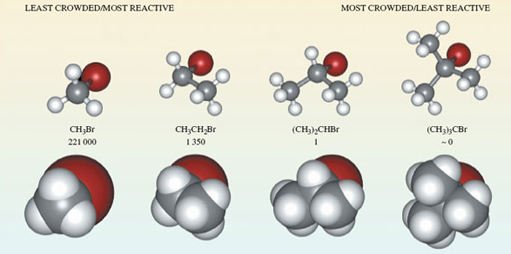
Ball-and-stick representations are a natural three-dimensional development of structural formulae, and they show the disposition of the atoms in space. But by emphasising the bonds, they fail to reveal the subtleties of the molecular shape created by the different sizes of atoms. In this respect, so-called space-filling models are better. These appear at the bottom of Figure 3. In each case, the viewing direction is the same as the ball-and-stick model above.
Note: also shown in Figure 3 are the relative rates of reaction of the four compounds when they are treated with lithium iodide in acetone (propanone) solution – you will be looking at this in the next section.