1.3.3 What is fission?
Fission is the splitting of large nuclei into smaller nuclei with the release of energy. Spontaneous fission is rare and generally fission is induced by bombarding the heavy nucleus with neutrons.
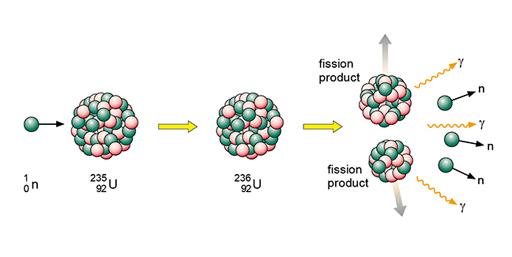
In the late 1930s it became evident that bombardment of uranium-235 by neutrons could lead to fission. The U-235 nucleus is able to absorb the neutron to become (very briefly) U-236. The U-236 then undergoes fission to form two new nuclei called fission products.
Fission also produces neutrons and energy. Neutron induced fission is illustrated in Figure 14. Bombarding the uranium-235 nucleus with a neutron leads to the formation of a uranium-236 nucleus, which very quickly undergoes fission. Fission products are formed, and neutrons are emitted. Note that gamma radiation is also emitted.
Neutrons are shown as using the same notation as for isotopes. The fission products themselves can vary but examples would be xenon-140 and strontium-93. An equation representing this particular fission would be:
In words this would be: ‘A uranium-235 atom absorbs a neutron to become uranium-236 which then undergoes fission to form the products xenon-140 and strontium-93 with three neutrons.’
Some important points to note:
- Fission products tend to be radioactive. These by-products of nuclear power form the majority of the radioactive waste that we will consider next week.
- The fact that neutrons are also produced means that these neutrons can go on to induce further fissions. Once started, fission can become self-sustaining – this is called a chain reaction.
- For fission to occur the neutrons must be going at the right speed – too fast and they will bounce off rather than be absorbed. The neutrons may need to be slowed down and are then referred to as thermal neutrons. Both the number and speed of the neutrons is crucial within a working reactor.
- The energy released per fission is relatively large. It is 50 million times more energy than burning the equivalent amount of carbon. We will consider where the energy comes from in the next section.
While uranium-235 is the isotope that undergoes fission it is worth noting that uranium-238 atoms can absorb neutrons to become plutonium-239 which is another atom that can undergo fission.