2.3 Ionic and covalent compounds – a comparison of properties
Consider a crystal of sodium chloride.
As a consequence of the strong attractive forces existing between the closely packed ions of opposite charge, the sodium chloride structure is not easily broken down: it has a high melting temperature and does not dissolve in organic solvents like the liquid hydrocarbons found in petrol. When it does melt, or dissolve in water, the ions separate and the resulting ionic fluid conducts electricity.
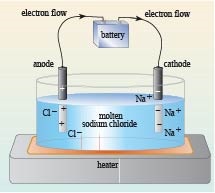
Figure 2 shows a simple experimental set-up to show molten sodium chloride (or indeed a solution of sodium chloride in water) conducts electricity. A battery is connected to two metal rods. The electrode attached to the negative terminal of the power source is called the cathode, and the anode is the electrode connected to the positive terminal.
Turning now to Cl2.
Here the bonding can be maintained only if the atoms stay together in pairs, so it gives rise to a molecular substance; as you saw earlier elemental chlorine consists of discrete Cl2 molecules with only weak forces acting between them. It is a gas at room temperature, and dissolves easily in liquid hydrocarbons, including petrol.
Would you expect a solution of chlorine in a hydrocarbon to conduct electricity?
No, because it contains no ions.
