2.1 Bonding in benzene
Benzene, a hydrocarbon is a one of the products of fractional distillation of crude oil, and the starting point for the preparation of many important chemical compounds. Superficially its molecular structure comprises six carbon atoms joined in a ring, but a closer look reveals some interesting points.
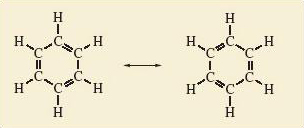
Benzene, like ozone, can be represented as a resonance hybrid of two resonance structures in which all atoms have noble gas configurations (Figure 5).
To dig deeper into the nature of the bonding in this molecule, let’s look at some simpler molecules.
Ethane has the formula C2H6 and comprises solely single bonds (C-C and C-H), whereas ethene (C2H4) contains a double bond between the two carbon atoms, and four C-H single bonds.
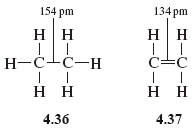
A typical C—C single bond length in ethane, (Structure 4.36), is 154 pm; in contrast, a typical C—C bond length in ethene, (Structure 4.37), is 134 pm. The individual resonance structures in Figure 5 therefore suggest that the carbon-carbon bond lengths in benzene should alternate between about 134 pm and 154 pm around the ring.
But what does the whole of Figure 5 suggest?
The real structure of benzene is a hybrid of the individual structures, and each carbon-carbon bond will be a mixture of one-half single and one-half double bonds; all carbon-carbon bond lengths should be equal and lie between 134 and 154 pm.
This is precisely the case: all carbon—carbon bond lengths in benzene are 140 pm!
Number the carbon—carbon bonds in a benzene ring of Figure 5 clockwise from 1 – 6. All bonds contain at least one pair of electrons. However, in one of the resonance structures, bonds 1, 3 and 5 are double bonds, each containing a second electron pair; in the other resonance structure, the double bonds and extra pair of electrons are found at bonds 2, 4 and 6.
The implication of Figure 5 is that in the resonance hybrid these three extra pairs of electrons are not confined to, or localised within, just half of the bonds in the ring. Instead, they are delocalised around the ring and equally shared within all six bonds.