3.2 Explaining molecular geometry?
To answer the question posed at the end of the last section,think about what you know about a chemical bond.
In a single sentence describe a covalent bond.
A shared pair of electrons holding together two atoms.
As bonds comprise negatively charged electrons, and two negatively charged objects will repel each other, logically you would expect bonds to repel each other.
As a result they adopt positions where they are as far apart in space as possible.
Consider now the molecule SF6 – sulfur hexafluoride, a potent ‘greenhouse gas’.
Looking simply at its forumula, you might expect SF6 would have the shape shown in Structure 6.3. The sulfur atom has six S—F bonds directed towards the corners of a regular hexagon, and all seven atoms are in the same plane. However experiment shows this isn’t the case and the actual shape is the octahedral one shown in Figure 7e.
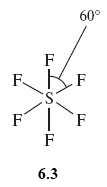
But, of course, you are aware of the nature of electrostatic repulsion so,
Suggest why SF6 is octahedral?
The S—F bonds repel one another, so that they get as far apart in space as possible. In Structure 6.3, they are confined to a single plane and the angle between them is only 60°. By adopting octahedral coordination, the greatest possible separation of the S—F bonds is achieved, the angle being increased to 90°.
All the shapes shown in Figure 7 conform to this principle by enforcing a good separation of the bonds in space. The principle looks even more reasonable if you recall that bonds comprise pairs of electrons; the like charges of these electron pairs lead to an expectation that one pair will repel another.
However, two very common molecules will soon dispel the notion that repulsion between bonding pairs of electrons is the sole determinant of molecular shape. These are water, H2O, and ammonia, NH3 and will be considered in the next section.
