Atomic structure theory
Let’s now look at another tricky topic. This time it is atomic structure theory. Again, this may not be a topic that you are familiar with but see if you can recognise the stumbling blocks.
Activity 2 Finding more stumbling blocks
a.
Understanding energy levels.
b.
Difficulty in explaining bonding and chemical reactions and how to predict reactants and products.
c.
Unable to grasp that subatomic particles are attracted and repelled by each other.
d.
Understanding space in atomic model.
e.
Difficulty in seeing trends and differences in groups and periods in the periodic table.
f.
Visualisation of atoms and subatomic particles.
g.
Difficulty using terminology to describe the subatomic model e.g. atomic number, relative atomic mass.
h.
Understanding charges in atoms.
i.
Inability to recognise charges of protons, neutrons and electrons.
j.
Difficulty seeing that atoms have different sizes and mass.
The correct answers are a, d, f and h.
Answer
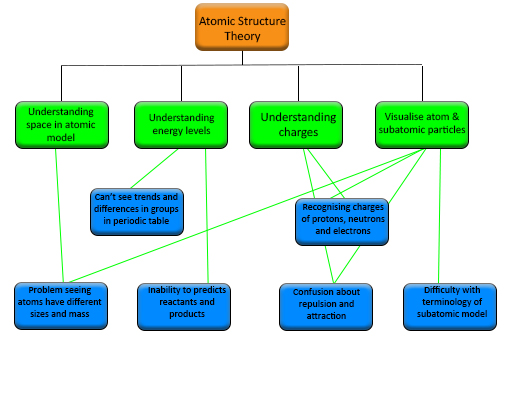
In Table 1 you see the stumbling blocks highlighted. You may have found it difficult to see them initially but once you see them highlighted they begin to make sense.
| SB1: understanding energy levels |
|---|
| Problem example: Difficulty in explaining bonding and chemical reactions and how to predict reactants and products. |
| Problem example: Unable to grasp that subatomic particles are attracted and repelled by each other. |
| SB2: understanding space in atomic model |
| Problem example: Difficulty in seeing trends and differences in groups and periods in the periodic table. |
| SB3: visualisation of atoms and subatomic particles |
| Problem example: Difficulty using terminology to describe the subatomic model e.g. atomic number, relative atomic mass. |
| SB4: understanding charges in atoms |
| Problem example: Inability to recognise charges of protons, neutrons and electrons. |
| Problem example: Difficulty seeing that atoms have different sizes and mass. |
Here is the structure chart for those stumbling blocks and problem examples in the atomic structure theory tricky topic. Unless you are a science teacher you would not be expected to be able to link the problem examples to all the stumbling blocks but this is something you should be able to do in your own subject.
This structure is becoming quite complicated isn’t it? In actual fact there are many more problem examples that define these stumbling blocks so you can imagine that if you tried to include them all, that this structure would become very complex indeed. In Section 3, you will find another structure which is used as part of the tricky topics process and will help you to structure your own tricky topic.