Introduction
The intention of this article is to provide some general knowledge about viruses, how they lead to infection and how the body’s immune system responds. Although tiny, viruses are complex, diverse entities that have the capacity to infect hosts, replicate themselves and then spread to additional hosts. In addition, viruses can sometimes transfer between host species, or they can mutate and re-infect the original host species.
What is a virus?
Viruses are microscopic packages of genetic information (DNA or RNA, depending on the type of virus), enveloped in a protein coat. They can vary in shape and size, and typically range from 20–300 nanometres (a nanometer is a meter divided by a thousand million) (Figure 1). Viruses can exist outside their host, but they can’t replicate in isolation, don’t have any of the cellular ‘machinery’ of a normal cell, nor any metabolism. Indeed, there is a debate as to whether viruses are actually a form of life and are sometimes considered to be at the ‘edge of life’. Like true lifeforms, viruses possess genetic material, can reproduce, and evolve through processes involving mutation and natural selection (albeit that the mutation requires host cells rather than occurring autonomously; unlike living organisms).
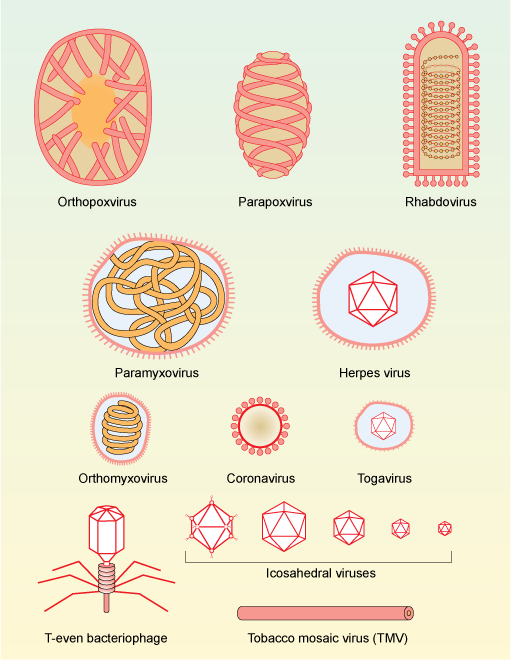
Figure 1. Morphology and approximate relative size of different families of viruses. Viruses consist of DNA or RNA that is often tightly associated with proteins to give more stability. The genetic material (DNA or RNA) can be contained within regularly-shaped proteins that give geometric shapes (capsids) or have a looser membrane covering, or both.
After infecting a host, a virus will enter the host’s cells (within a specific tissue or more generally, depending on the virus), and take over the cellular systems thereby turning the cell into a virus factory producing many new viral particles. This invasion and altered function can cause dramatic changes to the host’s cells, which can damage or kill them. The production of new viral particles leads to more of the host’s cells becoming infected and underlies the transmission of the virus to a new host.
It’s estimated that every millilitre of seawater contains up to 100 million viruses, while lake sediments contain around 20 billion viruses per gram.How long a virus can remain viable outside its host depends on the virus and its environment. Some viruses can survive in the air or on solid surfaces for several days or weeks. While viruses in water tend to survive for longer – up to a year in clean cold water. Heat, detergent, or UV light can inactivate viruses within minutes. By contrast, freezing temperatures can preserve viruses.
There are many different types of virus and they are everywhere. For instance, it’s estimated that every millilitre of seawater contains up to 100 million viruses, while lake sediments contain around 20 billion viruses per gram. Infected individuals can shed a lot of virus particles. Patients with the stomach bug, rotavirus, release up to 10 billion virus particles per gram of faeces.
The range of living organisms that viruses infect is vast. In fact, it is considered that all organisms, from unicellular entities such as bacteria, to more complex multicellular plants and animals, are susceptible to infection by different types of viruses. Some viruses specifically infect certain host species, whereas others infect a wide range of different species. The consequences of viral infection also vary enormously between particular host–virus interactions. Generally, although not always, viral infection results in disease of the host organism.
Where do viruses come from?
There are several theories regarding the origin of viruses. The fact that they infect all forms of life suggests that they have ancient origins.There are several theories regarding the origin of viruses. The fact that they infect all forms of life suggests that they have ancient origins. As mentioned above, viruses are essentially genetic material (DNA or RNA) surrounded by a protein coat. To be successful, the genetic material of viruses (commonly described as the ‘viral genome’) needs to encode for a sufficient number of genes such that a virus can achieve the cycle of infection–replication–re-infection. The amount of genetic information inside a virus is tiny. For instance, coronavirus genomes contain around 30,000 nucleotide bases, while the human genome is around 10,000 times larger, consisting of over 3 billion base pairs (‘bases’ and ‘base pairs’ are biology terminology referring to chemical structures in RNA and DNA; sequences of bases in RNA and DNA encode for all the proteins and determine the physical characteristics of organisms).
But, how did viruses come about? One idea is that viruses evolved from short sequences of genetic material that were originally part of a larger entity. It is suggested that these short sequences of genetic material, encapsulating a viable viral genome, escaped from the original cell. Indeed, it is known that some sequences of DNA have the ability to cut themselves out of a cell’s genome and reinsert into another part of the genome. If such ‘transposable elements’ were to become bound up in a piece of cell membrane and escape the cell, it could move to another cell – akin to how a virus would transfer. However, another view proposes that viruses are actually more ancient than other cells. This is because the majority of viral proteins have no similarity to proteins in any other organisms, suggesting their genomes are distinct and they pre-date cellular life. Because there are so many different types of viruses, it’s possible that both ideas are correct and different families of virus arose independently.
Different types of virus employ various replication strategies when infecting a cell. Retroviruses, for example, have an RNA genome. Retroviruses also possess an enzyme that can use the RNA template to produce DNA, which can then insert within the DNA of the host cell. This means that the infected cell and any of its progeny will contain viral DNA. An example of a retrovirus is human immunodeficiency virus (HIV). This process of viral genome integration into host cell genomes has been happening for millions of years and it’s estimated that around 8% or our DNA actually comes from viruses.
How do viruses infect us?
The surface of most viruses is studded with proteins that allow the virus particles to attach to host cells and gain entry. The interaction of viruses and host cells occurs through the interaction of specific virus and host cell proteins: blocking this interaction is a potential way for inhibiting viral infections. Once inside a cell, viral particles disassemble, and the viral genetic information becomes a template for the host cell to start making new viral proteins and genomes. New virus particles are either released from the host cell while it carries on functioning, or the host cell bursts and dies, thereby releasing the viral particles within.
How does the body respond to a virus?
Different species have evolved different strategies to overcome viral infections. In humans, the immune system can be classified into two types: innate and adaptive, both of which provide protection from viral infection. The innate immune system comprises physical barriers (e.g., mucous), chemical messengers (e.g., cytokines; a type of signalling molecule used by cells) and various types of cells (e.g., leukocytes; white blood cells) and is the first line of defence against invading microorganisms. A particularly important cytokine is interferon, which is released by cells when they are infected with virus particles. The interferon molecules released by an infected cell can activate signalling mechanisms in neighbouring cells to inhibit further viral infection. It has been suggested that cells infected with SARS-CoV-2 might not release interferons to the same degree as with other viral infections.
Unlike the innate immune system, the great advantage of the adaptive immune system is that it remembers the viruses and bacteria it has encountered.More targeted interventions against viruses come from the adaptive immune system. The adaptive immune system also involves leukocytes, such as the lymphocytes that recognise foreign proteins and produce antibodies. An antibody will bind to a specific region (known as an epitope) on a foreign protein, thereby allowing only the invading material to be targeted for clearance. The production of antibodies via the adaptive immune system can take several days to develop, so there may be a lag between the onset of infection and the ability to mount a response. However, unlike the innate immune system, the great advantage of the adaptive immune system is that it remembers the viruses and bacteria it has encountered. Consequently, if the body is exposed to the same pathogen again, it can rapidly increase production of those specific antibodies again. This immunity can diminish in time as the cells that remember the pathogen decline, which is why people might need to be re-vaccinated against a specific disease.
In order to prevent the spread of virus in our bodies, some infected cells undergo a specialised type of demise known as apoptosis (also called ‘programmed cell death’). Apoptosis is a physiological process and is a normal part of life that is used in a highly regulated way to remove damaged, unwanted or infected cells in all tissues. Cells infected with a virus can trigger apoptosis and thereby die. This sounds like a drastic course of action, but it effectively limits the continuous release of new virus from a cell.
Not all viruses are ‘bad’
In recent years, many bacteria have become resistant to antibiotics. Viruses that infect and destroy bacteria have therefore become a focus of medical research. These viruses, known as bacteriophages, do not infect human cells, so they can be used as selective antibiotic agents. Other viruses that selectively infect human cells can be used to deliver therapeutic DNA into patient cells, a technique known as gene therapy.
What do we know about the novel coronavirus that causes COVID-19?
COVID-19 is a disease caused by a newly recognised type of coronavirus called SARS-CoV-2. Theories have been proposed about how this novel coronavirus emerged, but more information is needed before its origin can be established. Other members of the coronavirus family include the viruses responsible for Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS) that also cause severe upper respiratory tract infections in humans. These same viruses can infect animals too. In chickens they also cause respiratory tract infection, while in cows and pigs they cause diarrhoea. Current data suggests that SARS-CoV-2 causes a lesser fatality risk (~1% of infected people die) compared to SARS (10%) and MERS (37%), but higher than flu (0.1%).
The surface of SARS-CoV-2 is covered with clusters of ‘spike’ proteins (Figure 2). These proteins specifically bind to a protein called angiotensin-converting enzyme 2 (ACE2), which is present on the surface of cells in the lungs and other organs such the heart, kidneys and intestines. Once attached, SARS-CoV-2 is internalised into the cell, the virus particle opens and releases its RNA genome, which is then used as a template to make more viral RNA and proteins. It has been found that cells involved in both the innate and adaptive immune systems can become rapidly depleted following SARS-CoV-2 infection, thereby severely compromising antiviral immunity.
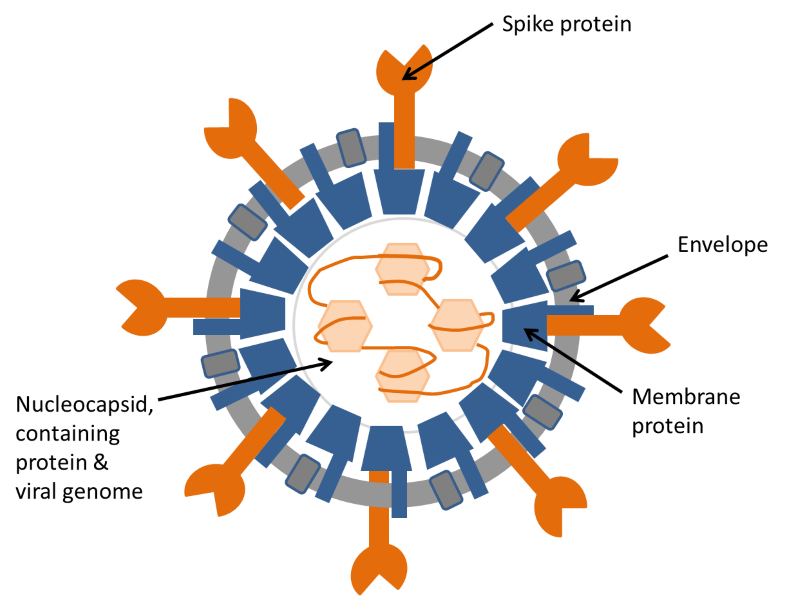
Figure 2. Diagram of coronavirus structure.
The illustration shows the structure of a coronavirus in cross-section. The coronavirus genome is a long strand of RNA wound around a protein and assembled inside a shell of membrane protein. This structure is surrounded by an envelope, containing two more proteins, one of which, the spike protein, is responsible for attachment of the virus to the target cell before infection. For more details see: https://www.ncbi.nlm.nih.gov/pubmed/22816037.
A considerable emphasis is currently being placed on mathematical models of SARS-CoV-2 transmission, which inform public health decisions to avoid overwhelming health care provision. Mathematical modelling is beyond the scope of this article, but the interested reader are referred to an informal video that explains a ‘SIR model’ (Susceptible, Infected, Recovered/Removed) of disease transmission that may apply to COVID-19: https://www.youtube.com/watch?v=k6nLfCbAzgo.
What is ‘herd immunity’?
A viral infection can only sweep through a population if individuals that are susceptible to it come into contact with viral particles. If people isolate themselves, then there’s much less chance of encountering the virus or passing it on to susceptible individuals. In addition, once someone has had a viral infection and has developed protective antibodies against it, they are generally not susceptible to re-infection. The more people within a population that have recovered from the virus, the less chance that the virus will encounter a susceptible person and the lower the chance that it will spread. This concept is known as herd immunity.
The proportion of the population that needs to be immune to infection in order to achieve herd immunity depends on several factors. A critical factor is the amount of contact between people since that can be how virus particles can spread. In a freely-mixing population, herd immunity depends on the number of people that, on average, become infected by one individual (known as the ‘basic reproduction number’; R0). The value of R0 varies depending on the type of virus. COVID-19 is estimated to have an R0 of around 2.5. By contrast, measles is much more easily spread, with an R0 of between 12–18. The proportion of the population that are required to be immune to a disease and thereby effectively stop its spread is known as the ‘herd immunity threshold’, and can be calculated using R0 in the following equation:
Herd immunity threshold = (R0 - 1)
R0
Thus, for COVID-19 with an R0 of 2.5, the equation would be:
CoVID-19 herd immunity threshold = (2.5 - 1)
2.5
= 0.6 (the same as 60%)
So, around 60% of the population need to be immune to COVID-19 in order to prevent further spread.
Herd immunity can be achieved through people becoming infected and their immune systems developing resistance, or through vaccinations using pieces of the virus that trigger a response from the adaptive immune system without resulting in disease. The more infectious a disease is, the higher the proportion of people who need to be vaccinated. In the case of measles, for example, which has a R0 of 12–18, the World Health Organisation recommends a vaccination rate of 95% to eliminate the disease, something that the United Kingdom is presently struggling to achieve.
At present there is no vaccine for COVID-19, which means that herd immunity could only come about by approximately 60% of the population becoming infected and developing immunity. For the United Kingdom, this would require close to 40 million people being infected for a herd immunity target to be reached. However, with a fatality risk of ~1%, many thousands of people would die from the infection. Moreover, many tens of thousands of the people who are infected would suffer from respiratory distress and require hospitalisation and those numbers, all at once, would overwhelm the health system. In the longer term, the world’s population may develop an immunity to COVID-19 that will mitigate further outbreaks, but herd immunity is not a plausible solution to the current COVID-19 crisis.
By producing different proteins, viruses can bypass the immunity gained via vaccination or a previous exposure to the original virus.It has been demonstrated that antibodies directed against the spike protein of SARS-CoV-2 can prevent the virus from entering cells. Such advances in our knowledge of SARS-CoV-2 will lead to the development of tests that show whether a person has been infected and is no longer susceptible as well as therapeutic vaccines.
One critical piece of information that is needed in the fight against COVID-19 is to know how much the SARS-CoV-2 virus genome can undergo mutation. A mutation would arise from a change of base sequences in its RNA genome, which would lead the production of a different protein. By producing different proteins, viruses can bypass the immunity gained via vaccination or a previous exposure to the original virus. It is known that SARS-CoV-2 can mutate, and indeed the virus that is currently spreading around the world would have undergone mutations as it transferred from its original species to humans. Moreover, it is known that there are genetic differences between the original SARS-CoV-2 that developed in Wuhan, China and the strains of the virus currently causing COVID-19 in the United States. This means that SARS-CoV-2 is mutating as it passes through human hosts. However, the good news is that the rate of SARS-CoV-2 mutation is not that high when compared to other viruses such as those that cause flu.
A substantial number of biomedical, pharmaceutical and clinical researchers have turned their attention to SARS-CoV-2. A quick check of research publication databases, such as PubMed, shows that there were 695 published items on Coronavirus in the whole of 2019. Whereas, the count is already 1,818 publications for the first 3 months of 2020. With each new bit of information, we get closer to understanding the virus and developing solutions to defend against it.






















Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews