4 Biological explanations for depression
Genetic, molecular and neuroimaging studies have continued to contribute to advances in understanding the neurobiological basis of depressive illness (Kupfer et al., 2012). Animal models for depression, and rodent models in particular (see Box 10), have been extensively used to test the efficacy of antidepressant medications leading up to clinical trials, and to inform and guide pharmacological therapies. Genetic variation in SLC6A4 (the serotonin transporter gene), FKBP5 (encoding a protein that helps regulate cortisol binding to the glucocorticoid receptor), TREK1 (a potassium channel mediating SSRI mechanism of action) and COMT (catechol-O-methyltransferase) amongst others, have been associated with the response to several antidepressant drugs in clinical studies.
Molecular studies also support at least three categories of biological determinants associated with depression:(i) reduction in neurotrophic and related factors such as BDNF, VEGF and IGF-1; (ii) excessive production of pro-inflammatory cytokines (immune mediators),including IL-1beta, IL-6 and TNF-alpha; and (iii) impaired regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis, which can be affected by antidepressant medication.
Box 10 Rodent models for depression
Despair-based: forced-swimming, tail suspension.
Reward-based: sucrose preference, intracranial self-stimulation.
Anxiety-based: novelty-induced hypophagia, open field, elevated plus maze, light/dark box.
Early life stress: maternal deprivation.
Stress-based models: learned helplessness, chronic mild stress, social defeat stress.
Others: genetically engineered mice; olfactory bulbectomy.
Neuroimaging studies have highlighted changes in neural systems involved in the processing and regulation of emotion, and in reward-seeking in people with major depressive illness. These include the amygdala and ventral striatum (brain areas that are involved in emotion and reward processing), medial prefrontal, orbitofrontal and anterior cingulate cortical regions (brain areas involved in ‘implicit’ regulation and processing of emotion), and the ventrolateral and dorsolateral prefrontal cortex (which are involved in cognitive control and the voluntary regulation of emotion) (see Figure 3).
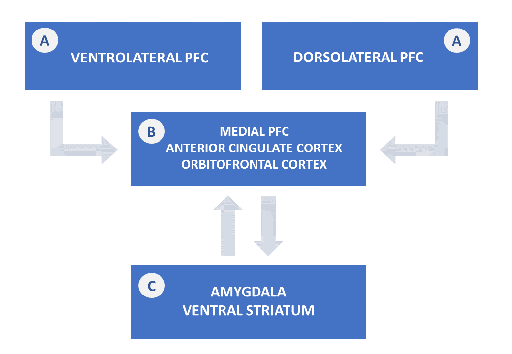
Functional connectivity between brain regions (the effect that activity in one region exerts over that in another) is altered in neural systems for emotion and reward processing and for regulation of emotion in major depressive disorder. The medial prefrontal-limbic neural network which regulates emotion (and includes the amygdala, anterior cingulate cortex and medial prefrontal cortex) is modulated by serotonin neurotransmission. The ventral striatum, and interconnected orbitofrontal and medial prefrontal cortices which are involved in reward processing, is modulated by dopamine.
People with major depression show a bias of attention towards negative emotional stimuli, and away from positive emotional and reward-related stimuli. Through measuring responses to fearful faces as a ‘negative stimulus’, activity within the amygdala, ventral striatal and medial prefrontal cortex was shown to be increased in people with major depression, whereas in response to positive emotional stimuli and receipt or anticipation of a reward, activity within the ventral striatum was atypically reduced in people with major depression when compared to those who were not depressed (see Kupfer et al., 2012).
Activity within the ‘default mode network’ (a neural system that involves several midline structures within the brain including the ventral medial prefrontal cortex, and can provide insight into brain function at rest and during self-reflection, associated with self-referential processing) is also altered in major depressive disorder.
Importantly, antidepressant treatment has been found to modulate activity within these neural networks: (i) reduced activity within the dorsolateral prefrontal cortex, anterior cingulate cortex and other regions implicated in cognitive control of emotion during the resting state in depressed individuals returned to ‘normal’ levels (i.e. levels observed in individuals who are not depressed) following treatment with antidepressant drugs; and (ii) overactivation of the medial prefrontal cortex and subcortical regions in response to emotional stimuli in the depressed state was also returned to normal after antidepressant treatment (Kupfer et al., 2012).
