7 'We are stardust'
How did the atoms in our bodies, the Earth, the Sun and other astronomical objects originate? This question is intimately tied with one of the most fundamental questions in science 'How was the Universe formed?'. Only the simplest atoms were present in the early stages of the Universe. Even now, the composition of normal matter in the Universe is dominated by hydrogen and helium. All the other heavier atoms, rather confusingly referred to as 'metals' by astronomers, amount to around 2% of normal matter. The heavier elements have been produced in nuclear reactions within stars.
If the nuclear reactions in stars occur close to the centre where the temperatures and pressures are highest, how can these heavy elements escape to make their way into the gas and dust clouds in the Galaxy and ultimately into planets and us? The answer lies in the details of the evolution of stars. The structure of stars changes as they age and some end their lives in catastrophic explosions that distribute much of their mass into the surrounding space and increase the fraction of heavier elements that can be incorporated into new stars.
About 1% of the Sun's photosphere is composed of heavy elements. If the Sun formed from clouds of gas and dust in the Galaxy why does it contain only half the amount of heavy elements now present in the Galaxy?
The Sun formed when the Galaxy was considerably younger (around 4.6 billion years ago). As time passes more and more stars complete their life cycles and so the proportion of heavy elements increases. The Sun therefore formed when there were fewer heavy elements in the Galaxy.
Table 2 lists the fraction by mass of the most common elements in the photosphere of the Sun compared with those in the Earth and a human body. As you would expect, the elements that are most common in the Sun are those that are most commonly produced in the nuclear reactions in stars. However, if the Earth formed at around the same time as the Sun why does it have such a different composition? The most abundant atoms in the Universe are only minor constituents of the Earth. The answer lies in the way in which these elements are combined in molecules that formed the building blocks of the planets.
| Abundance (% by mass)* | |||||
|---|---|---|---|---|---|
| Element | Sun (photosphere) | Whole Earth | Earth's crust | Human | |
| 1 | Hydrogen | 74 | 0.1 | 10 | |
| 2 | Helium | 25 | |||
| 8 | Oxygen | 0.6 | 30 | 47 | 61 |
| 6 | Carbon | 0.2 | 0.1 | 0.1 | 23 |
| 26 | Iron | 0.1 | 32 | 5.1 | |
| 10 | Neon | 0.1 | |||
| 12 | Magnesium | 0.1 | 15 | 2.1 | |
| 7 | Nitrogen | 0.1 | 2.6 | ||
| 14 | Silicon | 0.1 | 16 | 28 | |
| 16 | Sulfur | 0.6 | 0.1 | 0.2 | |
| 28 | Nickel | 1.8 | |||
| 20 | Calcium | 1.7 | 3.7 | 1.4 | |
| 13 | Aluminium | 1.6 | 8.1 | ||
| 11 | Sodium | 0.2 | 2.8 | 0.1 | |
| 24 | Chromium | 0.5 | |||
| 15 | Phosphorus | 0.1 | 0.1 | 1.1 | |
| 25 | Manganese | 0.2 | 0.1 | ||
| 17 | Chlorine | 0.1 | 0.1 | ||
| 27 | Cobalt | 0.1 | |||
| 19 | Potassium | 2.6 | 0.2 | ||
| 22 | Titanium | 0.1 | 0.6 | ||
Footnotes
* Abundances of less than 0.1% are not shown. The biggest contributors are in bold. Totals do not add up to 100% because of rounding and missing elements.The Earth is a rocky body, so elements that form minerals and rocks (oxygen, silicon and metals such as iron and magnesium) are the most common. Helium is an inert gas (as are neon and argon), which means that it does not react with other atoms to form molecules, and therefore does not contribute significantly to the rocky material of the Earth. The gas giant planet Jupiter has a composition much closer to that of the Sun; its vast atmosphere is composed of mainly hydrogen and helium.
The elemental composition of the human body is dominated by hydrogen, carbon and oxygen, three of the four most abundant atoms in the Sun. Water is formed from hydrogen and oxygen, and carbon is the key to forming highly complex molecules. These organic (carbon containing) molecules provide the framework for constructing the complex structures present in the human body and for carrying the information that allows humans to grow and reproduce (one of the definitions of life).
Helium is the second most abundant element in the Universe but is not a significant component of the human body. Why is this?
Because helium is an inert gas it does not form into molecules that are present in the human body.
It is beyond the scope of this short discussion to investigate the requirements for these complex molecules that are essential for life (as we know it) to exist. Nevertheless understanding how those conditions are satisfied on the Earth is the first step in attempting to find environments elsewhere where life may exist.
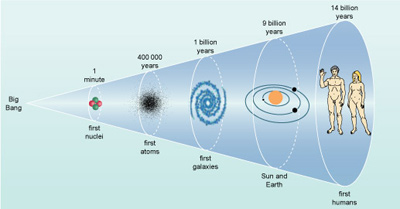
Current views of different parts of the Universe offer a wonderful spectacle, but all astronomers know that, as they peer across vast cosmic spaces, they also look back over great reaches of cosmic time. This is an unavoidable consequence of the finite speed of light. The light seen today from the most distant observable galaxies was emitted over 12 billion years ago. The earliest signals of any kind that can be detected (a particular kind of microwave radiation that is the remnant of the Big Bang that is believed to have formed the Universe) originated over 13 billion years ago. The Sun and Earth were formed around 4.6 billion years ago, and life has developed on Earth within the last 3 billion years. Only in the last million years of this vast timescale, did humans evolve on Earth (Figure 17), and only in the last century have they been able, in theory, to communicate their presence to other possible inhabitants of our galaxy. One of the most exciting recent developments in astronomy has been the detection of planetary systems around other stars. Many astronomers believe firm evidence for the presence of extraterrestrial life, either on another planet in our Solar System or orbiting a different star, will be found during our lifetimes. The prospects for finding extraterrestrial intelligent life appear to be much more remote.