Practice Problems Answer key
Only open this once you've completed the Practice Problems assignment.
All answers are in red text.
Solutions to Practice Problems for Biochemistry, Session 2: Covalent Bonds, Hydrogen Bonds
Question 1
Consider the following carbohydrate.
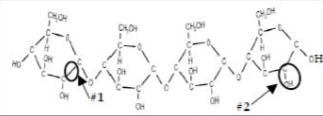
a) Name each bond or interaction that is circled in the schematic above. Explain why you selected these options. Choose from polar covalent, nonpolar covalent, ionic, hydrophobic, van der Waals.
• Bond #1 (C-C):
Non-polar covalent because carbon always forms covalent bonds in biological systems
and both carbon atoms have the same electronegativity making the bond non-polar
• Bond #2 (C-OH):
Polar covalent because carbon and oxygen have different electronegativities. Oxygen
is much more electronegative than carbon and pulls the electrons more towards itself
making the bond polar.
b) Would you expect this polymer to be soluble in an aqueous environment? Explain your answer.
Yes, because of the exposed OH groups that allow for hydrogen bonding with water in the environment.
c) What type of bonds or interactions can occur between two such polymers? Choose from: covalent, hydrogen, hydrophobic, ionic, van der Waals. Explain your answer.
Hydrogen bonds can occur between the two polymers. The exposed OH groups allow for
easy hydrogen bonding between molecules. There are no charged groups so ionic bonds
cannot occur and covalent bonds cannot occur without an enzyme or catalyst.