Unit 1 Final Exam
For this exam, you will need to know the properties of amino acids, nucleic acids, and phospholipids, as well as bonds, polarity, respiration, and photosynthesis.
Write all your answers down on a separate piece of paper or digital document.Biochemistry Unit Exam
Question 1
a) An example of a structural representation is shown in the lower box. Draw a structural representation of the amino acid, Aspartic acid, which has the side chain of: CH2COOH.
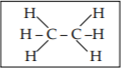
b) The molecule drawn above is a monomer found in _________________.
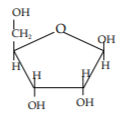
c) Below is a pentose found in RNA. On the diagram circle the hydroxyl that differs between ribonucleotides and deoxyribonucleotides.
d) You find an organism that lives in thermal pools with temperatures as high as 160 °F. You find that many of this organism’s proteins have a high percentage of cysteine. Briefly explain why this might be the case.
e) To the right is an iconic view of a phospholipid.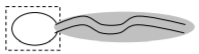
• List what atoms are found in a phospholipid molecule represented by the boxed region of this schematic. Describe the properties associated with this region.
• List what atoms are found in a phospholipid molecule represented by the shaded region of this schematic. Describe the properties associated with this region.
f)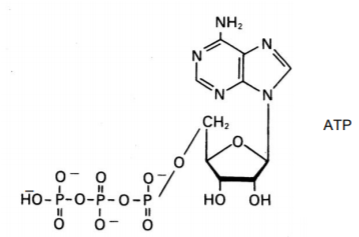
a) Box the part that is added to a growing chain of nucleic acid.
b) Star the atom(s) that can form a hydrogen bond with the complementary nitrogenous base.
c) Circle the part of the molecule that decreases the stability of RNA as compared to DNA.
d) Draw an arrow to the part of this molecule that you would modify to prevent further elongation. Indicate what change you would make next to the arrow drawn.
Question 2
Shown below is the structure of Drug 1, that binds to the E. coli ribosome. Indicate whether each circled region could possibly form ionic bonds, hydrogen bonds, or hydrophobic interactions with another molecule by filling out the table below. Fill in the table with “yes” if that type of bond is possible, “no” if it is not.
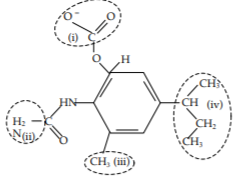
a)
| Part | Could this part form Ionic Bonds | Could this part form Hydrogen Bonds | Could this part be involved in a Hydrophobic Interaction |
|---|---|---|---|
| (i) | |||
| (ii) | |||
| (iii) | |||
| (iv) |
A representation of the drug bound to the E. coli ribosome is shown below. Three amino acids of the ribosome that are important for binding are shown. If this drug can bind to different bacterial ribosomes, it may be useful as an antibiotic. The ability of this drug to bind to the ribosomes of two different species is examined. The drug binds to the ribosome of species 1, but does not bind to the ribosome of species 2.
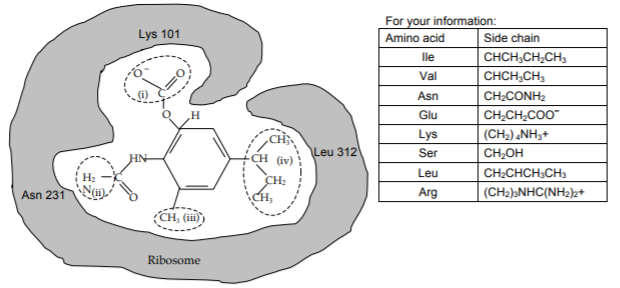
b) The table shows the differences between the ribosomes. Given this information, complete the table indicating which would be from species 1 (drug binds) and which would be from species 2 (drug does not bind).
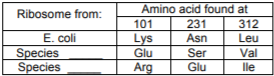
c) What specific amino acid or amino acids prevents the ribosome from species 2 from binding Drug 1?
d) In terms of the specific non-covalent interactions, explain why the ribosome from species 2 will NOT bind Drug 1, but the ribosomes from E. coli and species 1 will.
Question 3
Reaction 1) A![]() B, where ΔG’ = +2
B, where ΔG’ = +2
a) The energy profile for the reaction 1 is drawn on the axes below.
• Label A, B, and ΔG’ on the graph below.
• What is the value for the energy of activation?
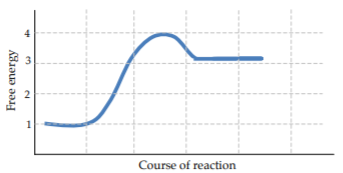
Compare reaction 1 (above) to reactions 2 and 3 (below).
Reaction 2) C![]() D, where ΔG’ = -3, Energy of activation = +0.5
D, where ΔG’ = -3, Energy of activation = +0.5
Reaction 3) E![]() F, where ΔG’ = +0.3, Energy of activation = +2
F, where ΔG’ = +0.3, Energy of activation = +2
b) Which of these reactions, 1, 2, or 3 is most likely to proceed in the forward direction in the absence of an enzyme? If you do not have enough information to answer this question, write “Can’t tell” below. Explain your answer.
c) When an appropriate enzyme is added to each of these reactions, the rate of the reaction increases. Which reaction will proceed the fastest? If you do not have enough information to answer this question, write “Can’t tell” below. Explain your answer.
d) Assume that you have a reaction X Y where ΔG = 0 and the energy of activation = +0.6 kcal/mole. In a cell, you find that the reaction proceeds almost exclusively in the forward direction. Explain why this might be the case.
Enzyme 1 carries out the reaction: W → X + Y. There are three regions where enzyme 1 interacts with substrate W (another protein) to facilitate the conversion of W into X and Y.
Region 1 is stabilized by hydrophobic interactions between the side chains of the amino acids found on the enzyme and substrate.
Region 2 is stabilized by hydrogen bonds between the side chains of the amino acids found on the enzyme and substrate.
Region 3 is stabilized by ionic bonds between the side chains of the amino acids found on the enzyme and substrate.
e) You have the following pairs of amino acids. For each pair given, list in which region (1, 2, or 3) this pair would act to stabilize the interaction of enzyme 1 with substrate A. If the pair could be found in more than one region, list all that apply. If the pair would not be found in any region write in the word NONE.
Pair
Amino Acid on Enzyme
Amino Acid on Substrate
In what region or regions would
the pair be found?
1
alanine
leucine
2
arginine
isoleucine
3
serine
methionine
4
threonine
serine
5
lysine
arginine
6
glutamine
asparagine
7
proline
valine
8
aspartic acid
lysine
Question 4
You inoculate two test tubes with the same amount of identical growth medium and with the same number of identical yeast cells and grow these cells under identical conditions except for the presence or absence of oxygen.
a) After 12 hours, all of the glucose in each culture has been consumed. You determine the number of total cells found in each culture and find that one culture has more cells than the other.
• Which culture would have the greater cell density, the one grown aerobically or the one grown anaerobically?
• Explain why the culture you chose above can make more cells with the same amount of glucose than the other culture.
b) The cells of both cultures convert glucose to pyruvate via glycolysis, and then further metabolize pyruvate.
• Are the cells of both cultures able to obtain the same amount of ATP from glycolysis? Explain.
• Under anaerobic conditions, the carbon from pyruvate will ultimately be found in which molecule?
• Under aerobic conditions, the carbon from pyruvate will ultimately be found in which molecule?
c) Under aerobic conditions, when glucose is metabolized some of the energy is used to reduce NAD+ to NADH + H+ . In the mitochondria, NADH donates its electrons to NADH-Q reductase, and from there the electrons move through the electron transport chain.
• Explain why this process requires aerobic conditions.
• Briefly describe how the transfer of electrons from one protein to another in the electron transport chain results in the production of ATP.
d) In oxygenic photosynthesis, electrons from the chlorophyll found in photosystem II are donated to the primary electron carrier. How are the donated electrons replaced?
e) Anoxygenic photosynthetic organisms make ATP using an electron transport chain, but do not produce O2 as a waste product. What is a common source of electrons for the conversion of NADP+ to NADPH for in this type of organism?
f) Circle all of the metabolic processes that occur in organisms that perform oxygenic photosynthesis.
Glycolysis Citric acid Cycle Oxidative phosphorylation
Calvin Cycle Cyclic photophosphorylation Non-cyclic photophosphorylation
g) Both respiration and photosynthesis are evolutionarily conserved. Based upon the current understanding of how life on earth evolved, which of these processes likely evolved first? Justify your answer in the space below.