Practice Problems Answer Key
Only open this when you've completed the Practice Problems assignment.
Correct answers are in red text.
Practice Problems for Biochemistry , Session 4: Proteins, Levels of Structure, NonCovalent Forces
Question 1
You have discovered a new enzyme, enzyme E, which breaks down proteins by cleaving peptide bonds after tyrosine or phenylalanine.
a) Enzyme E is the product of gene G that encodes a protein with the molecular weight of 50 kilodaltons (50 kD). When you purify enzyme E, you obtain a single type of polypeptide of 50 kD. However, active enzyme E has a molecular weight of 250 kilodaltons (250 kD), not 50 kD.
i) Why might active purified enzyme E be larger than the product encoded by gene G?
The active enzyme must have multiple subunits. Likely it is a pentamer of the 50 kD polypeptide encoded
by gene G.
ii) Define primary, secondary, tertiary, and quaternary structure.
The primary structure is the linear sequence of amino acids.
The secondary structure is localized regions of alpha helix and beta sheet.
The tertiary structure is the 3 dimensional shape.
The quaternary structure is the association of distinct polypeptide chains with each other.
iii) Is the primary structure of the 50 kD protein the same or different than the primary structure of the 250 kD protein? Explain briefly.
Because each subunit is encoded by the G gene, the linear sequence of amino acids is the same.
Thus the primary structure is the same.
iv) Is the tertiary structure of the 50 kD protein the same or different than the tertiary structure of the 250 kD protein? Explain briefly.
It is likely that the tertiary structure is the same. Each polypeptide folds to form the same 3 dimensional
shape. However, if the association between the subunits influences the shape of each, the tertiary
structure could be different.
v) Is the quaternary structure of the 50 kD protein the same or different than the quaternary structure of the 250 kD protein? Explain briefly.
The 50 kD protein, as a single polypeptide does not have quaternary structure, the quaternary structure of
the 250 kD protein is the five subunit nature of this protein.
b) You test enzyme E activity on a large protein substrate. This substrate is not cleaved by enzyme E. You then treat the substrate with DTT (a compound that disrupts disulfide bonds) and test the enzyme E activity again.This time the substrate is cleaved by enzyme E. Why was enzyme E able to cleave the protein substrate only after the substrate was treated with DTT?
Enzyme E binds and cuts at specific sites. These sites are not present on the exterior of the substrate when the substrate is properly folded. When the disulfide bonds within the substrate protein are disrupted, the 3 dimensional shape is altered, and the protein unfolds. This allows enzyme E access to sites that were previously protected within the substrate protein.
Question 2
For the first pair of amino acids listed below, draw the two amino acids with the side chains interacting and list the strongest type of interaction that can occur between the side chain groups. For the remaining pairs, simply list the strongest type of interaction that occurs between the side chain groups. Choose from covalent bonds, hydrogen bonds, ionic bonds, or van der Waals interactions.
i) tyrosine, asparagine hydrogen bond
ii) cysteine, cysteine covalent bond
iii) isoleucine, valine van der Waals
iv) glutamic acid, lysine ionic bond
v) glycine, glutamine van der Waals
Question 3
In analyzing differences between drug resistant fungi and drug sensitive fungi, you have discovered a protein that exists only in the drug resistant fungi. You named this the Resist protein and design substrates that you hope will bind to it.
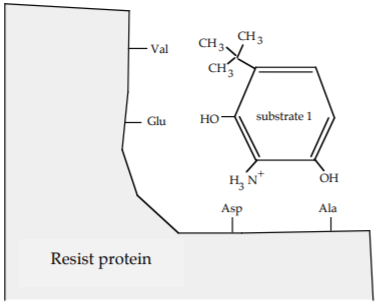
a) Give the name for the strongest intermolecular interaction between substrate 1 as shown and the side chains of following amino acids on the Resist protein. Choose from ionic bond, covalent bond, hydrogen bond, and van der Waals forces.
| Amino Acid | Strongest Interaction |
|---|---|
| Val | van der Waals forces |
| Glu | hydrogen bond |
| Asp | ionic bond |
| Ala | van der Waals forces |
b) You make the following additional substrates .
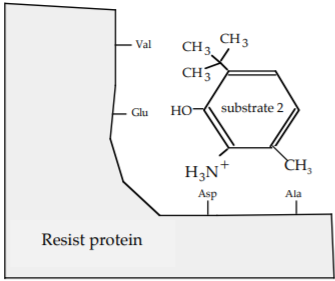
What is the strongest interaction that now exists between the Ala of the Resist protein and substrate 2?
Van der Waals force
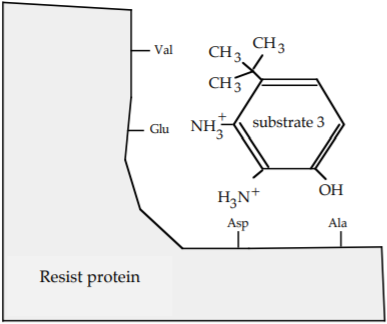
What is the strongest interaction that now exists between the Glu of the Resist protein and substrate 3?
Ionic Bond
c) Which substrate would you expect to bind the most tightly to the Resist protein?
substrate 1 substrate 2 substrate 3
Explain why you made this choice.
Substrate 3 forms strong ionic interactions at two positions as opposed to one position.