3.1 Radiometric dating of Moon rocks – some background
Before discussing the dating of samples using radioactive decay, it’s worth recapping a bit of background science. If you’re familiar with these concepts you can skip quickly through this step and the next.
Radioactive decay is the process whereby an atom decays to form a different element. One of the most commonly used methods for dating rocks on both the Moon and the Earth is the decay of an isotope of potassium (K) to produce an isotope of argon (Ar). It’s not like nuclear fission where the whole atom breaks up releasing lots of energy, but it is a natural process going on all around us and even in your body. Naturally occurring potassium has two stable isotopes and one unstable isotope and in this case, scientists make use of the unstable one that has been slowly decaying through time.
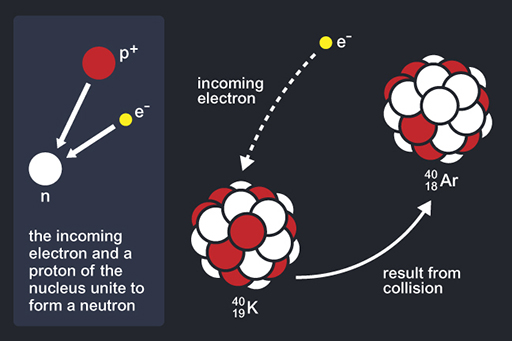
So, what is an isotope? Atoms are made of a cloud of electrons (negatively charged particles) surrounding a nucleus of protons (positively charged particles) and neutrons (neutral particles). Each element is defined by its atomic number, the number of protons in the nucleus (Z). To keep an atom electrically neutral overall, the number of electrons is the same as the number of protons. However, the number of neutrons (N) can vary in the atoms of a single element, resulting in atoms of different masses for the same element. The atomic mass (A) is defined by the number of particles in the nucleus:
A = Z + N.
Thus, isotopes of an element have the same value for Z but different values for A. In the case of potassium-40, you have Z = 19, N =21 and so A= 40. From now on, we won’t show the Z values.
