3 Biomass as a fuel
Fuels are materials from which useful energy can be extracted. In using biomass as fuel, this release of energy usually involves burning – known as combustion.
Some essential features of combustion are:
- it needs air – or to be more precise, oxygen
- the fuel undergoes a major change of chemical composition
- heat is produced, i.e. energy is released.
Consider for example, methane – the principal component of the fossil fuel natural gas and also one form of gaseous biofuel. As shown in Figure 4.4, each methane molecule consists of one carbon and four hydrogen atoms: CH4. Atmospheric oxygen has molecules consisting of two atoms (O2). In combustion, each methane molecule reacts with two oxygen molecules to produce carbon dioxide and water. The heat energy released is the difference between the chemical energy of the original fuel plus oxygen, and the chemical energy of the resulting carbon dioxide plus water. It is the energy content (or heat content) of the methane.
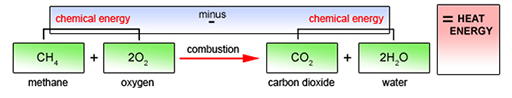
Table 1 shows the energy content of the crop produced by burning one tonne, or one cubic metre, of various biological materials.
| Category | Major energy-rich components | Structural strength/resistance to natural decay | Examples | Typical yields of dry matter /t ha-1 y-1 |
| Woody biomass | Lignin/lignocellulose (complex carbohydrates) | High | Trees (deciduous or hardwoods) | 10 (temperate) to 20 (tropics) |
| Cellulosic biomass | Lignin/lignocellulose (complex carbohydrates) | Medium | Grasses (e.g miscanthus), water hyacinth, seaweeds | 10 (temperate) to 60 (tropical aquatics) |
| Starch/sugar crops | Simpler carbohydrates | Low | Cereals (maize, sugar cane, wheat) | 10 (temperate cereals) to 35 (sugar cane) |
| Oily crops | Lipids (i.e. oils/fats) | Low | Oilseeds (rape, sunflower, oil palm, jatropha) | 8 to 15 |
| Micro organisms | Oils | Low | Microalgae | Unknown – still speculative |
