4 Plant nutrients
Certain inorganic substances are essential for normal plant metabolism but they can reach such levels as to be considered pollutants.
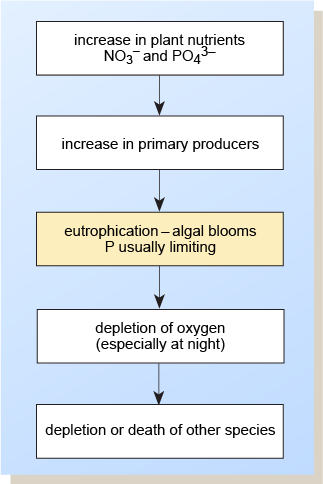
Eutrophication is the increase with time of plant nutrients and biota in a watercourse. Farming contributes 50–60% of nitrates and 20–30% of phosphorus getting into UK waters (GOV.UK, n.d.), with most of the remainder coming from treated sewage effluent. Pollutants from these sources can greatly accelerate the natural process by increasing the inputs of nutrients so that algae grow rapidly and algal blooms may form (Figure 5). The resulting rapid removal of carbon dioxide by photosynthesis can upset the bicarbonate–carbonate equilibrium and cause a pH rise, which in itself is damaging to the ecosystem.
In addition, at night the excess respiration (without the replacement of oxygen by photosynthesis) can deplete oxygen reserves, causing the death of higher organisms such as invertebrates and fish. This process can be compounded when algal blooms, through their decay, further reduce the oxygen content of the water.
In shallow water, the formation of benthic (bottom-living) mats of algae can create a smothering layer over sediments. This can hinder the supply of oxygenated water to eggs and impede the emergence of fry from salmon spawning grounds, for example.
Of the plant nutrients, the total inflow of phosphorus and nitrogen (especially at the productive time of year, spring and summer) is the most important. The main types of organism associated with algal blooms, and the different conditions needed for their continued growth, are as follows.
Blue-green algae (cyanobacteria)
Blue-green algae are able to fix atmospheric nitrogen and therefore are not limited by nitrate levels in the water. Neither are they dependent on dissolved carbon dioxide, because they can use the bicarbonate ions present. In addition, they are tolerant of relatively high pH. Their growth is limited by the phosphorus content.
Unicellular green algae
Green algae require nitrate, as they are unable to carry out nitrogen fixation. They also require fairly high levels of carbon dioxide, as they cannot use bicarbonate ions, and they are not tolerant of high pH values. Their growth is limited by both phosphorus and nitrogen.
Contact with or ingestion of blue-green algae can cause skin rashes, eye irritation, vomiting, fever, and pain in the muscles and joints, due to toxins produced by the algae. In addition, ‘red tides’ are harmful algal blooms that appear in coastal areas. They can produce toxic effects in humans, marine organisms and birds. The toxins produced may also make the surrounding air difficult to breathe (NOAA, 2013), and the bloom of algae often turns the water red.
Generally, increases in both phosphate and nitrate concentrations seem to be the most important factor in governing the rate of eutrophication in most waters. However, whether this is the only factor that limits the rate of eutrophication is still a matter of controversy. Any eutrophication is contingent on other aspects of the particular ecosystem such as the hardness, the pH value and the original distribution of algal species.
For freshwater plants, about eight times more nitrogen is required than phosphorus. Phosphorus thus limits eutrophication if nitrogen is more than eight times as abundant as phosphorus, while nitrogen limits eutrophication if its concentration is less than eight times that of phosphorus (UNEP, n.d.).
Nitrates can have a more significant effect on human health than phosphates if they are present in drinking water supplies.
- When nitrates are ingested by infants under six months of age, they can be converted to nitrite by bacteria in the digestive system. Nitrites combine with haemoglobin in the bloodstream, preventing it from carrying out its normal function of combining with oxygen and carrying it around the body. This can result in a serious, though rare, condition called methaemoglobinaemia (‘blue baby syndrome’), which can be fatal (Skipton and Hay, 1998).
- Ingestion of nitrate and nitrite by people with a low intake of vitamin C increases the risk of stomach cancer (Ward et al., 2011).
Water quality standards exist to control pollution of domestic water supplies. The maximum limit set by the EU for nitrates in drinking water is 50 g m−3 (as NO3−) (EU, 1998). Water with a high nitrate content can be treated for drinking using reverse osmosis.
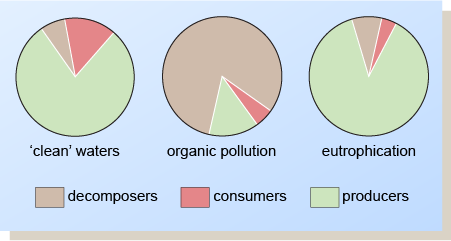
The effects on the biota of organic pollution and artificial eutrophication are summarised in Figure 6. When organic pollution occurs, the main types of organisms present are the decomposers. In the case of eutrophication, the producers are dominant, and in larger quantities than in ‘clean’ waters.