3 Organic materials
Organic substances constitute the major freshwater pollutants, coming from domestic sewage discharges (even after treatment) and from certain industries such as food processing. This section will deal with the biodegradable forms, but there are also inert (non-biodegradable) toxic forms. Organic substances can be natural (in which case they are normally biodegradable) or synthetic (in which case they can often be degraded by microorganisms that have adapted to utilising them).
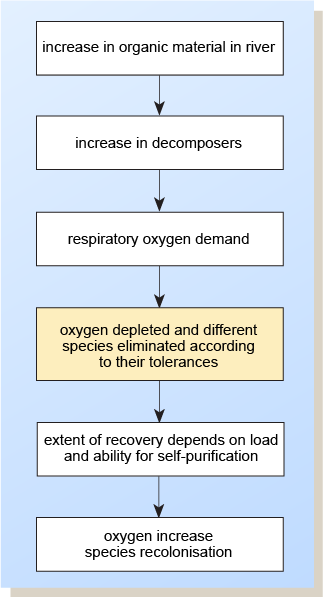
The major polluting effect of biodegradable organic materials is the reduction in oxygen concentration in the water. Bacteria and other organisms (decomposers) break these materials down into simpler organic or inorganic substances. They use up oxygen in the process, and as their population increases there is an extra demand for dissolved oxygen.
When a potentially polluting effluent is released into a stream, there follows a sequence of events in time and distance. This sequence leads to different environmental consequences and different aquatic communities compared with those immediately upstream and the successive reaches downstream. After a certain distance, natural biodegradative processes will break down the pollutants, often returning the river to something like its original condition.
Three stages of organic pollution can be defined.
- When the load is small, there will be little change in the species of plants and animals present in the water and little variation in the natural cycles. Initially, the dissolved oxygen will be near to saturation level. Any organic pollution apparent at the point of discharge will disappear within a short distance downstream as it is removed by the natural processes of self-purification. It could be said that, in some instances, mild organic pollution is beneficial to the river, since it increases the nutrient supply for microorganisms present in the natural state. This minimal pollution can benefit the whole aquatic ecosystem.
- If the load increases, the dissolved oxygen level will drop significantly and the river will be polluted for a considerable distance from the point of discharge. Some species of animals and plants will flourish at the expense of others. In the absence of further pollution, the river will probably recover downstream, but if the area around the discharge remains polluted then this can act as a barrier to the passage of migratory fish (among other disadvantages).
- If the polluting load is increased still further, the natural ecosystem will be grossly distorted and its effectiveness in coping with the pollutant load greatly reduced. The level of dissolved oxygen will be very low or fall to zero. Often the only organisms to flourish will be sewage fungus, certain worms and fly larvae. (These organisms give a low Biological Monitoring Working Party (BMWP) score, indicating the polluted nature of the river.) Also, anaerobic bacteria may thrive and give a foul smell to the water by metabolising organic substances and producing methane, hydrogen sulfide and ammonia. Few algae are able to thrive under severe organic pollution, so reoxygenation by photosynthesis will be hindered. The river will now remain polluted for a much greater distance downstream.
The sequence of events following significant pollution of a waterway by organic material is shown in Figure 4.
Inorganic materials can also cause deoxygenation, e.g. when ferrous iron from mine drainage water enters a river (Stumm and Lee, 1961). In the reduced ferrous (Fe(II)) state the iron is in solution, but on meeting the oxygen in the river it is oxidised to red insoluble ferric (Fe(III)) iron, a process that reduces the concentration of dissolved oxygen in the river water. The oxidised iron is now in suspension so that, as well as reducing the oxygen content, it reduces light penetration. It finally settles out slowly downstream of the discharge point, giving rise to all the problems associated with suspended solids. This type of problem is usually associated with coal-mining effluents.
Activity 1
a.
a. Depletion of oxygen occurs because of an increase in the activities of primary producers in both cases.
b.
b. In both cases it is the presence of plant nutrients (nitrates and phosphates) that causes the death of green plants and depletion of oxygen.
c.
c. The difference in the proportion of producers and consumers between organically polluted waters and eutrophic waters is negligible.
d.
d. A reduction of dissolved oxygen in both cases causes the depletion of species.
e.
e. Once the dissolved oxygen content is decreased, only the removal of the offending pollutant can allow an increase in species.
The correct answers are d and e.
Answer
Statements (d) and (e) are true.
Statement (a) is false: depletion of oxygen occurs in both cases due to the activities of decomposers. An increase in primary producers (plants) would increase oxygen levels.
Statement (b) is false: only in eutrophication are plant nutrients present, and these will usually encourage plant growth.
Statement (c) is false: in organically polluted waters the producers are only a small proportion of the ecology, making up less than 25% of the population, whilst in eutrophic waters they constitute more than 75%. The proportion of consumers, on the other hand, is similar in both situations (though slightly greater in organically polluted waters).