1 Natural waters
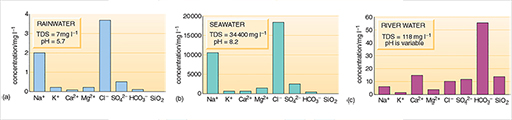
Rainwater, seawater and river water (Figure 2) and groundwaters (Figure 3) generally have very different chemical compositions and differ widely in their concentrations of total dissolved solids (TDS). Average TDS values are: 7mg l-1 for rainwater, 118mg l-1 for river water and 34 400 mg l -1 for seawater. TDS values for groundwater vary too much for an average to be meaningful. TDS is a good indicator of water quality, and standards that have been set for drinking water and for water used in other ways include maximum values for TDS.
Rainwater and seawater (Figure 2a and b) have similar relative proportions of dissolved solids, although rainwater is much more dilute. Most of the dissolved salts in rainwater come from sea spray dispersed into the atmosphere. A major difference in composition is the greater relative proportions of dissolved gases in rainwater, particularly carbon dioxide. Natural rainwater is slightly acidic as a result of this reaction, with an average pH of 5.7, whereas the average pH of seawater is 8.2 (Box 1). Rainwater may be even more acidic in areas where the highly soluble acidic gases sulphur dioxide and nitrogen dioxide (both produced by fossil fuel power generation, transportation and industrial processes) are present in the atmosphere.
Box 1 pH
pH is a measure of how acidic or alkaline a solution is. The pH scale ranges from less than 1 to 14, with low values the most acid, and high values the most alkaline. A neutral solution (or pure water) has a pH value of 7.
An acidic solution has a higher concentration of hydrogen ions, H+, than pure water (this is where the 'H' in the term 'pH' comes from) and a pH of less than 7. For example, when carbon dioxide (CO2) dissolves in water, a slightly acidic solution is formed:
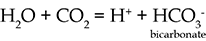
The pH of most natural waters lies between 5.5 and 8.5.
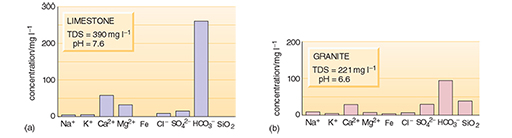
River water and groundwater differ from rainwater in that both have greater TDS values and different relative proportions of dissolved substances (Figures 2c and 3). Rivers may also contain solid particles in suspension, in addition to dissolved substances. Groundwater usually has a low content of suspended solids because these are filtered out as the water passes through the ground. Organic processes in soils, the solution of soluble minerals in rocks, interaction with clays and other minerals, and the chemical weathering of rocks are responsible for the changes in composition as rainwater becomes surface water or groundwater. The relative proportions of the dissolved substances change and the TDS value increases as a result of these processes. In general, groundwater takes on the chemical properties of the rocks through which it passes.
Activity 1
What are the three principal dissolved constituents of river water and both groundwaters in Figures 2c and 3?
Answer
For the river water and granitic groundwater the three most abundant are bicarbonate (HCO3-), calcium (Ca2+) and silica (SiO2). For the limestone groundwater, bicarbonate and calcium are again abundant, but magnesium (Mg2+) is more abundant than silica (although this may not be the case for all limestones).
The main reason for the abundance of HCO3- and Ca2+ is the solution of calcium carbonate (CaCO3) which can be present as limestone or as a cement in sandstone. Limestone is a fairly common rock, and it dissolves readily in acidic waters such as rainwater:
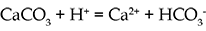
The mineral dolomite (CaMg(CO3)2) has a similar reaction in acidic water, and provides a source of magnesium ions. Magnesium also comes from the weathering of minerals such as olivine and pyroxene. Silica comes from the weathering of silicate minerals, which are a major constituent of most common rocks.
Box 2 Hardness in water
Hardness in water is mainly due to the presence of ions of the elements calcium, magnesium and iron. High concentrations of these ions have objectionable side-effects, particularly scum and scaling. The ions react with soap, forming insoluble compounds and preventing the soap from lathering properly, causing rings on bathtubs and leaving a grey soap scum on washed clothes. Hard water also leaves mineral deposits (limescale) in plumbing and appliances that use water, particularly kettles.
If the hard water contains bicarbonate ions, carbonate salts of the metals are precipitated when the water is boiled or heated above 70°C. Such water is said to possess temporary hardness because the carbonate salts are largely insoluble and are thus removed from the water as limescale deposits.
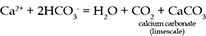
When the main anions (negatively charged ions) present are chloride, sulphate or nitrate, the hardness is called permanent hardness, which cannot be removed by boiling.
Some people in hard water areas use water softeners, which usually replace calcium, magnesium, and certain other ions in the water with sodium ions. The sodium ions are supplied from common salt (sodium chloride) in the water softener. While softened water containing sodium ions in moderate concentration is unobjectionable in taste or household use, it may be of concern to those who, for health reasons, are on diets involving restricted sodium intake.
In spite of the problems encountered when boiling and using soap in hard water, the dissolved solids may give hard water a pleasant taste and have various medicinal benefits.
The extent of hard water in Britain tends to follow a north to south-east gradient; the softest water is in Scotland, northern England and Wales, and the hardest is in East Anglia and south-east England. Death from cardiovascular (CV) disease (heart disease and stroke) tends to follow a similar pattern — a higher rate in the north and north-west than the south and the south-east. Several statistical surveys in a number of other countries have also shown this inverse relationship between CV disease and water hardness, i.e. CV disease is more common in softer water areas. However, there is no evidence of a direct causative link between CV disease and soft water, but as a precaution standards have been set for water that has been artificially softened. The UK has set restrictions on levels of water softening, and UK drinking water should now have a minimum hardness, if it has been softened, of 60 mg l -1 of Ca2+.
The composition of water will vary with the type of rock the water has flowed over or through. Water flowing through igneous and metamorphic rocks usually has lower TDS values than that flowing through sedimentary rocks, because igneous and metamorphic rocks contain minerals that are generally less soluble. Water that has flowed through deposits of ore minerals will often have a high metal ion content and may have a high sulphate content, derived from sulphide ore minerals.
The TDS value of the water also depends on the length of time the water has been in contact with rock. Groundwaters usually have higher TDS values because the residence time of groundwater is generally higher than that of surface water. The composition of groundwater changes as it passes through an aquifer. Near the recharge area, groundwater has low TDS values, but as the water flows through the aquifer it gains more dissolved substances, so the TDS values are usually higher at discharge points.
In hot dry regions where rainfall is low and evaporation is high, there is little infiltration and the soluble products of chemical weathering are flushed from aquifers very slowly. So groundwaters can have high TDS values in these areas.
Deeper groundwaters that are slow moving often have particularly high concentrations of dissolved substances with TDS values exceeding a few thousand milligrams per litre. Sodium chloride is usually a major constituent of these waters. When these saline waters are discharged at the surface they are often called mineral waters (Figure 4).

Activity 2
Looking back at Figure 3, why does the groundwater from the limestone area have a higher TDS value than the groundwater from the granite area?
Answer
Minerals in the limestone are much more soluble than the minerals in granite, especially if the water is slightly acidic. The high TDS is caused mainly by calcium and bicarbonate ions.
The variation in natural waters may make it hard to determine when water is polluted. Although pollution means a deterioration in water quality caused by human agencies, the same effect may occur naturally. For instance, large amounts of sediment and vegetable matter can be washed into rivers during rainstorms; toxic metals and acid waters can get into rivers and groundwaters where concentrations of ore minerals occur; and contamination can result from oil seepages at the surface. These are all natural processes, and many of the effects will be neutralised in time; the environment has ways of adapting itself in the long term. Pollution, on the other hand, can be on a very large scale, can happen rapidly, can take a variety of forms, and can upset the ecological balance where it occurs.
