
According to this table aluminium should react with oxygen more readily than iron, which is easily oxidized in the presence of water and oxygen. How is it then that aluminium can be used for aeroplanes which are in constant contact with water and oxygen?
In reality, aluminium does react with oxygen very rapidly and forms aluminium oxide, Al2O3. The oxide has the ability to adhere to the surface of the underlying metal with great tenacity .
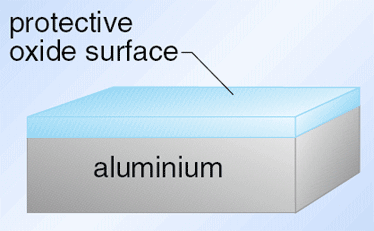
This thin but robust layer of oxide then protects the underlying metal from further oxidation
If the oxide becomes scratched and aluminium metal exposed, more oxide is formed and the protected layer renewed. Even if aluminium metal is cleaned with emery paper, a protective oxide layer would form before the metal could be put into the solution containing Fe2+(aq) ions.
The situation with iron is very similar in the sense that iron oxidizes and forms the familiar red–orange rust. However, rust takes up a greater volume than the iron from which it is formed and consequently splits and cracks. It is also porous to water and oxygen so that more rust can be formed from the underlying metal and eventually the oxidation process is complete.
Moisture, in addition to oxygen, is also necessary for the rusting of iron. Rust is not the simple oxide Fe2O3 but is a compound that actually contains water molecules. It can be represented by Fe2O3.nH2O where n can vary according to the conditions under which the rust forms. The water molecules are incorporated into spaces within the crystal structure of iron oxide.
Iron (and steel) is not weather-resistant yet it does find widespread use because of its relative cheapness and its mechanical properties. Perhaps the most obvious way in which steel can be prevented from rusting is to protect the metal surface with a coating of something that does not rust. The most widely used coating is paint and it is very effective provided that the paint does not become damaged. If this happens, or the metal surface is not completely free of rust when it is painted, then further rusting can occur .
The situation is made worse because rusting can spread under the paintwork and is often at an advanced stage when signs of blistered paint become apparent. Where a hard paint surface is not required (such as the inside of car body panels), waxes and paints that do not set completely are useful. If such a surface suffers a small scratch, the paint will flow a little and effectively self-heal the scratch.

Another coating that protects steel is to use a metal that is further down the activity series, a metal that does not oxidize in the air. The ideal metals are gold, silver and copper, which are towards the bottom of the activity series.
| Metal | Reactivity |
|---|---|
|
caesium |
high
|
Galvanized buckets and dustbins that used to be commonplace did not rust even though they were made from steel. The metal surface of these containers appears to have irregular shiny crystals.
Galvanizing is the process by which a metal surface is given a coating of zinc. Zinc melts at 420°C, and objects to be galvanized are chemically cleaned and then dipped into a bath of molten zinc. The process of galvanizing does seem to work; it is more expensive than conventional paint protection but is finding increasing use in the motor industry.
Where does zinc come in our activity series? We have argued that to coat steel with a metal that does not oxidize in air makes sense, so it seems odd to use a metal that oxidizes even more readily than does iron.
Zinc, like aluminium, oxidizes easily. It also has an oxide that clings well to the metal and is not porous to water or oxygen. So the surface of the steel is protected by zinc metal overlain with zinc oxide. But there is a further advantage to using zinc. If the zinc coating is damaged and iron exposed, one might expect the iron to rust just as if a protective paint surface had been damaged. However, as zinc is more easily oxidized than iron, zinc is oxidized in preference and the iron remains in the metallic form as we can see here.
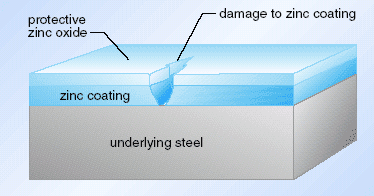
Representation of the protection of a galvanized surface. Electrons produced when zinc is oxidized to Zn2+ inhibit further oxidation of the underlying iron to the metal cations.

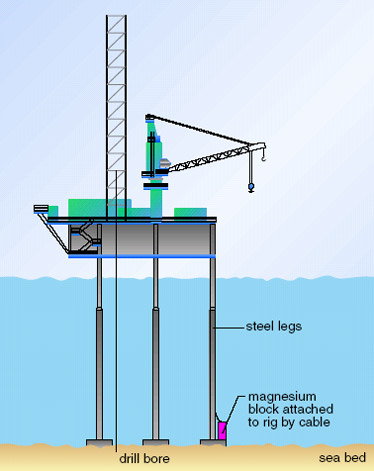
A related protective method is used for larger steel structures such as buildings, ships and underwater/underground pipelines. For such large structures, it would not be practical to provide a covering of zinc. What is done is to attach a block of an easily oxidized metal (magnesium is often used) to the structure by a steel cable
Magnesium preferentially oxidizes to form the Mg2+ ion:
Mg(s) = Mg2+(aq) + 2e-
The electrons produced can flow along the steel cable and give the oil rig a small negative charge. This makes it difficult for iron in the structure to oxidize and form positively charged ions as this would add even more electrons to what is an already negatively charged structure and the steel rig remains intact. In time, the block of magnesium is eaten away and has to be replaced. This is much easier and cheaper than carrying out repairs on the structure of the rig.
Modifying metals
The resistance to corrosion of a metal can be changed by providing a surface coating of paint or other metal, but there is another way of changing the properties of the metallic elements that has been known for thousands of years. Bronze is an alloy of copper and tin, and like copper, its major component, it is resistant to corrosion. Bronze is also much harder than is copper and it can be used in engineering. It finds particular use in the manufacture of propellers for ships where corrosion resistance is paramount. Here, there is a further advantage: copper is toxic to molluscs and is effective in inhibiting the growth of barnacles on the propeller which would reduce its operating efficiency .
An alloy is different from a chemical compound which has a fixed ratio (and usually a very simple one) of one element to another. We have seen that the relative proportions of copper and tin in bronze can be varied over a wide range. For any ratio, atoms of the two metals are evenly distributed through the structure and the properties of the overall structure are very dependent on that ratio. The hardness, strength and corrosion resistance of metals can often be ‘tuned’ by carefully adjusting the proportions of elements in the alloy.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews