3.2 Get a half-life!
An unstable isotope decays over time at a rate that is characteristic of the particular isotope and is proportional to the number of surviving atoms. The result is that the number of atoms falls exponentially or undergoes exponential decay. A key feature of exponential decay is this: whatever number of atoms you start with, the time taken for half of them to decay will always be the same. This time is called the ‘half-life’ of the particular isotope.
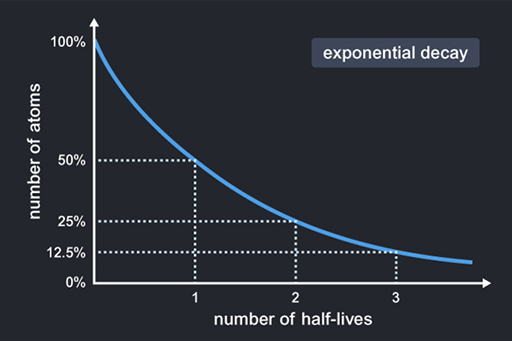
Exponential decay allows scientists to use the amount of surviving isotope to measure the ages of rock and minerals. The most commonly used dating technique for Moon rocks uses an unstable isotope of potassium (40K or potassium-40) that decays to a stable isotope of argon (40Ar or argon-40). The decay rate is very slow, even on the long timescales of the history of the Moon. Half the potassium-40 atoms decay in 1250 million years and this time span is called the half-life of potassium-40. So there have been about 3.6 half-lives of potassium-40 since the formation of the Moon. That means there is only about 8% of the original potassium isotope remaining, as you can see on the graph. This technique is known as K–Ar dating.
The other key difference between the elements potassium and argon is that argon is volatile and escapes when rocks are melted or very hot, but potassium is not volatile and so tends to be retained. So, for example, the atoms of argon escape from lavas when a volcano erupts, so when things cool down afterwards, the ratio of potassium to argon is higher than before. When the rocks like those from the lunar maria cool, they begin to retain argon and the amount of argon increases as potassium in the rock continues to decay. Thus the K–Ar decay clock is reset by an eruption. Another example is an event such as a large asteroid impact on the Moon’s surface causing melting of the surface rocks; this also preserves the age of the impact. By resetting the decay clock, scientists measure the amounts of both the potassium and argon isotopes in rocks using sophisticated measurement techniques and thus estimate the age of geologically important events in the far distant past.
The K–Ar dating technique was applied to Moon rocks when they were returned to Earth by the Apollo missions and is the reason why we know so much about the Moon’s history. We know that the Late Heavy Bombardment caused massive cratering of the Moon’s surface between 4.1 and 3.8 billion years ago because many of the lunar highland rocks were heated and melted then. We also know that most of the lunar maria erupted between around 3.8 and 3.6 billion years ago because they cooled and began to retain argon in that period.
