2.2 Markers of life
Metabolic processes are very specific chemical reactions. The existence of life in a location means that these reactions change the environment and influence the types of chemicals found.
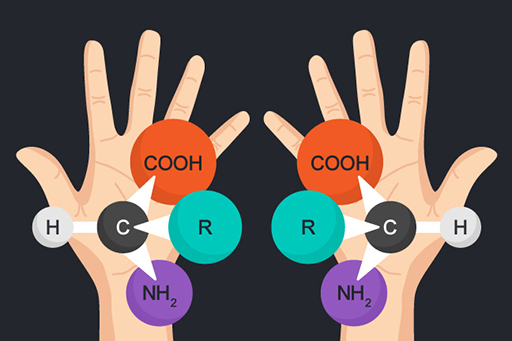
One example of this is organic molecules that have the feature of chirality, also called handedness. Such ‘chiral molecules’ come in two forms: the left-handed form and the right-handed form. The chemical compositions of the two forms are identical but the structures are such that a right-handed molecule is a mirror image of a left-handed molecule and one cannot be superimposed on the other. Glucose is a well-known example of a chiral molecule. For a familiar macroscopic example of chirality, just look at your hands: they are mirror images of each other. It is impossible to hold them with palms facing in the same direction in a way that one hand covers the other. Chemical reactions that are associated with life have the tendency to prefer one mirror image over the other, while non-biological reactions have no such bias. In consequence, in a life-free environment the right-handed and left-handed versions are present in equal amounts. If life is present, one form of a chiral molecule will be much more abundant than the other.