5 Conclusion
We have seen in this course that, despite having a high natural abundance, iron is in very short supply because of the insolubility of its oxides and hydroxides. A result of this is that organisms have developed methods for the uptake, transport and storage of iron. Bacteria, in particular, secrete very powerful iron chelators known as siderophores. Of all the iron–siderophore complexes, the iron(III)–enterobactin complex has the exceptionally high stability constant of 1049 mol−1 l.
Other organisms, partly as a defence against siderophores and the need to avoid free iron in solution, have biochemical methods to transport and store iron. The protein most associated with iron transport is transferrin. Iron storage, in mammals, including humans, is achieved by ferritin, which stores iron as a hydrated iron(III) oxide; this is an example of biomineralisation.
The key points of this course are:
The concentration of free Fe3+(aq) at pH 7 is very small.
Free Fe2+(aq) in an organism is dangerous, because it can react to produce the superoxide radical anion.
Iron(III) tends to be coordinated by hard ligands such as those containing oxygen or nitrogen.
The chelate effect confers extra stability on iron(III) complexes with polydentate ligands.
Siderophores are powerful microbial iron chelators.
Enterobactin is a particularly powerful iron chelator It acts as a preorganised hexadentate ligand for iron(III).
Esterases catalyse the hydrolysis of the triserine ring in enterobactin, thus enabling iron(III) from the enterobactin complex to be released.
Iron transport in mammals is carried out by transferrin, which holds iron(III) in a hexacoordinate site, one of the ligands being η2-carbonate.
Ferritin is an iron storage protein capable of storing up to 4 500 iron atoms per protein macromolecule. The iron is stored as hydrated iron(III) oxide.
SAQ 2
What is a siderophore?
Answer
A siderophore is a microbial iron chelator, whose complex with iron (III) usually has a very high stability constant. Examples include enterobactin, aerobactin and agrobactin.
SAQ 3
Compounds 38 and 39 are synthetic siderophores. The stability constant of the iron–38 complex is 1040 mol−1 l, whereas the stability constant of iron–39 is 1028 mol−1 l. Explain why both stability constants are less than that for iron-enterobactin, and why the stability constant for iron–38 is greater than that for iron–39.
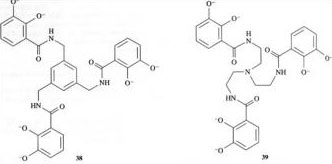
Answer
As with enterobactin, both 38 and 39 will coordinate to iron(III) via the six deprotonated oxygens in each structure (highlighted in green below).
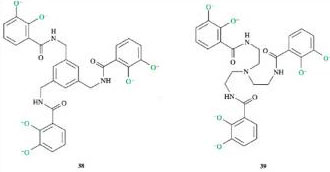
The key feature of 38 and 39 that distinguishes them from enterobactin is the replacement of the triserine ring with 1,3,5-trimethylbenzene in 38 and a triethylamine in 39. The effects are twofold. Firstly, both 38 and 39 are not as rigid (preorganised) as enterobactin. Secondly, both 38 and 39 have different relative spacing of their catechol groups compared to enterobactin. In fact, on chelation of iron(III), the catechol groups in 38 and 39 cannot obtain the most stable spatial arrangement around the iron, because their motion is restricted by the 1,3,5-trimelhylbenzene in 38 and the triethylamine in 39. Hence the stability constants of the complexes formed by 38 and 39 with iron(III) are lower than for enterobactin. 38 has a slightly higher stability constant with iron(III) than 39 for two reasons. Firstly, 38 is slightly more rigid than 39 and not so much ligand reorganisation is required on complex formation. Secondly, the size of the trimethylbenzene is slightly larger than that of the triethylamine, and the catechol groups in 38 are able to approach the most stable spatial arrangement around the iron(III) ion better than in 39.
SAQ 4
Structure 40 is the siderophore agrobactin. Sketch the conformation you would expect it to adopt in the complex it forms with iron(III), and indicate the location of the iron(III) binding site.
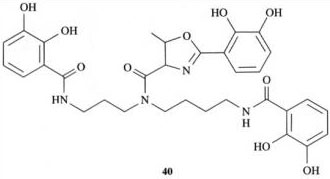
Answer
Structure 44 shows the expected conformation of agrobactin in its iron(III) complex, where the iron is chelated by the three deprotonated catechol groups, in an analogous fashion to the iron(III) binding with enterobactin.

