2 Principles of iron chemistry
2.1 The problems of iron uptake
Iron has a high natural abundance. It is the second most abundant metallic element by mass in the Earth's crust (7.1 per cent).
Activity 1
What are the main oxidation states of iron?
Answer
Naturally occurring iron exists primarily in two oxidation states, +2 and +3, but in the presence of O2 the most stable oxidation state is +3.
The iron concentration in seawater is very low, roughly 5 × 10−11 to 2 × 10−8 mol l−1
Activity 2
If an aqueous pale-green coloured solution of iron(II) nitrate, Fe(NO3)2, at pH 7 is exposed to air, a brown-coloured precipitate soon forms on standing. The precipitate, which contains iron(III), settles to the bottom of the vessel. What do you think has happened?
Answer
The precipitate is mostly hydrated iron(III) oxide, Fe2O3.nH2O (rust).
So, how is the hydrated iron(III) oxide formed? In aqueous solution, iron(II) will react with O2 to give iron(III):
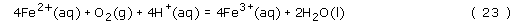
The iron(III) is a strong Lewis acid, and will react with water in the following series of hydrolysis reactions to give highly insoluble Fe(OH)3 and Fe2O3.3H2O, which appear as a brown-coloured precipitate:
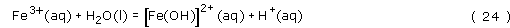
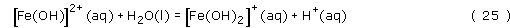
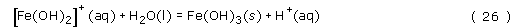

Notice that the hydrolysis reactions must be pH dependent, since a proton is produced in each of the reactions. Therefore, at low pH, say less than pH 1.5, iron(III) is soluble in water, but at pH 7 insoluble Fe(OH)3 is formed, Fe(OH)3 is profoundly insoluble, with a solubility product, Ksp = 2 × 10−39 mol4 l−4.
Activity 3
What is the concentration of Fe3+(aq) in H2O at pH 7, assuming that Fe(OH)3(s) is present?
Answer
We know that the solubility product, K sp, of Fe(OH)3 = 2 × 10−39 mol4 l−4. Therefore,
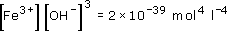
At pH 7: [H+] = 10−7 mol l−1 and [OH−] = 10−7 mol l−1. (Remember that we calculate this from Kw = [H+][OH−] = 10−14 mol2 l−2.) Therefore,
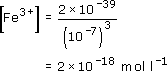
From the above calculation, we can see that at pH 7, the concentration of Fe3+(aq) is extremely small: 2 × 10−18 mol l−1. The value of 5 × 10−11 to 2 × 10−8 mol l−1 for Fe3+(aq) in seawater is much higher than this.
Activity 4
Why do you think the concentration of Fe3+(aq) in seawater is higher than the value you have just calculated?
Answer
This is due to the presence of other ligands that can form soluble iron complexes. For example, chloride, bromide, acetate and nitrate are all potential ligands.
Nevertheless, for such an important bioinorganic element, the concentration of Fe3+(aq) in neutral aqueous solution is very low indeed. This very low concentration is a significant problem when it comes to an organism obtaining iron, since iron in its soluble form is in such short supply. If an organism is to survive, it must have some biochemical means of absorbing the trace amounts of surrounding iron. Also, the organism must prevent iron(III) oxide precipitation once the iron is inside the organism, since this may lead to cell damage. (Although having said this, some organisms, such as pigeons, are known to crystallise iron oxide deliberately within certain parts of their bodies, particularly the brain. These small iron oxide pellets are often magnetic and are thought to act as in-built compasses.)
Activity 5
What are the products of the reaction of a single iron(II) ion with a single molecule of O2?
Answer
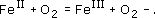
The reaction of O2 with iron(II) gives single-electron oxidation of the iron(II) to iron(III) and the generation of a superoxide radical anion.
This anion is highly toxic to biological systems and must be avoided where possible. Therefore, any free iron(II) is potentially detrimental, acting as a reducing agent for O2.
Free iron(II) dissolved within an organism is also potentially dangerous. Therefore, the organism must have methods for preventing the formation of free iron(II). (Note It is now easy to see why iron-overload patients suffer from a variety of secondary diseases, since the extra iron is not controlled by the normal biochemical systems that deal with iron. There is an increased availability of free iron, which can be chelated by micro-organisms, and an elevated level of iron(II).)
The last property of iron we shall examine is the thermodynamic stability of its coordination complexes.
Activity 6
Would you classify iron(III) as a hard or soft metal?
Answer
Iron(III) is a first-row transition metal in a high oxidation state and so is classified as a hard metal. As such, it will tend to form stable complexes with hard ligands. Typical hard ligands contain oxygen and nitrogen as the coordinating atoms.
Activity 7
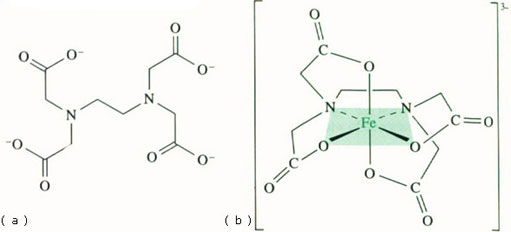
Would you expect iron(II) or iron(III) to make a more stable complex with the edta4− anion (Figure 2a)?
Answer
The hexadentate edta4− ligand coordinates through both O and N. The stability constant of the [iron(III)–edta]3− complex (shown in Figure 2b) is c.1025 mol−1 l, whereas the analogous stability constant with iron(II) is only c. 1014 mol−1 l.
Complexes containing chelate rings are usually more thermodynamically stable than similar complexes without rings; for instance, log10(stability constant) for [Ni(NH3)6]2+ is 8.61, whereas that for [Ni(en)3]2+ is 18.18. This is known as the chelate effect. It is observed for pairs of complexes when the coordinating atom of a monodentale ligand, L, is the same as that of a bidentate ligand, L—L, and there is no steric strain in the chelate ring. It is thought that several factors are involved overall in the chelate effect, but that the most influential is the entropy change in the formation reaction. This can be seen in the experimental data given below in Table 1 for the formation of two four-coordinate cadmium complexes:

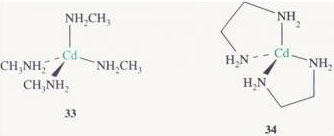
[Cd(CH3NH2)4]2+, 33, is coordinated through nitrogen to four monodentate methyl-aminc ligands, whereas [Cd(en)2]2+, 34, is coordinated through nitrogen to two bidentate ethylcnediamine ligands. We see that the chelated complex is far more stable than the complex with no rings — by a factor of over 104 in this case.
| Complex | Log10 (stability constant, K) | ||||
|---|---|---|---|---|---|
| [Cd(CH3NH2)4]2+ | 6.55 | -57.32 | -37.41 | -66.8 | 19.91 |
| [Cd(en)2]2+ | 10.62 | -56.48 | -60.67 | 14.1 | -4.19 |
It is evident that the enthalpy change for the formation reaction of each complex is very similar, which is to be expected because the atoms involved in each bond, and therefore type of bonding, are similar in each case.
Activity 8
What do you notice about the values for the change in entropy?
Answer
They are very different. The entropy change for the chelated complex is positive, whereas that for the complex with no chelate rings, is negative.
The large difference in entropy changes, ![]() , leads to a big difference in the Gibbs free energy changes for the reactions.
, leads to a big difference in the Gibbs free energy changes for the reactions.
Activity 9
How does this lead to a difference in the stability constants of the two complexes?
Answer
From the relationship ![]() , we see that a positive entropy change will lead to a lower or more negative value for the Gibbs free energy change
, we see that a positive entropy change will lead to a lower or more negative value for the Gibbs free energy change ![]() , of the reaction.
, of the reaction. ![]() , so the more negative is
, so the more negative is ![]() , then the larger is K and the more stable is the complex.
, then the larger is K and the more stable is the complex.
The leading question now is, why does this happen? The answer seems to be that the dominating factor is the entropy of the ligands. Consider what happens when a hexa-aquo metal ion, M, reacts with a complexing monodentate ligand, L:
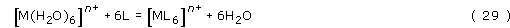
there is no change in the number of molecules in solution: the six water molecules are displaced by six ligand molecules.
Activity 10
What is the significant difference when the same reaction takes place with bidentate ligands?
Answer
In a similar reaction with bidentate ligands, L–L,
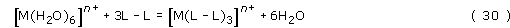
there is a net increase of three molecules in solution.
For gas–solid reactions, a greater increase in the number of gas molecules in a reaction leads to a greater entropy change. Something similar happens here, but in solution. The increase in the number of independent molecules in the chelate reaction leads to a more positive ![]() .
.
The chelate effect is usually at a maximum for five- and six-membered rings: smaller rings tend to suffer from strain, and in larger rings the second coordinating atom is no longer very close to the metal.
Macrocyclic ligands, such as porphyrin rings, tend to show the same stabilising effect as polydentate ligands and this is sometimes known as the macrocyclic effect.