4.3 Iron storage
In humans, iron is stored mainly in the bone marrow, spleen and liver. About 10 per cent of all the iron in the body is in storage. Two proteins are involved in iron storage; these are called ferritin and haemosiderin (they also occur in other organisms). We shall only study the better characterised (and simpler!) ferritin.
Each ferritin molecule can store iron up to about 20 per cent of its total mass. This is a very high percentage, considering that less than 0.2 per cent of the total mass of proteins like transferrin and myoglobin is iron. Ferritin is a large protein with a relative molecular mass of 440 000. The crystal structure of ferritin with no iron is shown in Figure 10. The overall structure shows that ferritin is a huge, hollow protein, with a wall mostly made up of α-helical peptide chains. The structure is quite symmetrical, being roughly dodecahedral, and is one of the outstanding examples of symmetry in chemistry. The wall contains channels, which lead from the inside to the outside of the hollow ‘sphere’. The channels are rich in amino acids with carboxylate side-chains, which are capable of chelating iron.
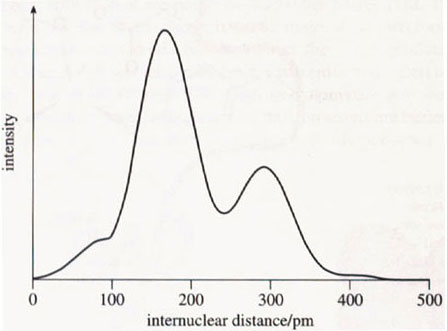
The crystal structure of iron-containing ferritin is not known. However, some clues as to its structure have been obtained from EXAFS studies. EXAFS gives information about the direct coordination environment of a particular atom in terms of the number and type of its coordinated atoms (although no angular information is usually available). EXAFS studies on iron-containing ferritin showed that each iron atom is surrounded by an inner shell of six or seven oxygen atoms at a distance of about 160 pm, and by a second shell of seven or eight iron atoms at a distance of about 290 pm (Figure 11). This was a very strange result. How could seven or eight iron atoms be packed around each iron atom? The problem was solved when it was noticed that the EXAFS data were very similar to that of a hydrated iron(III) oxide mineral called ferrihydrite, 5Fe2O3.9H2O. From this result it was clear that ferritin stored iron partly as a crystalline, hydrated iron(III) oxide. Further studies showed that the inorganic crystalline part was within the hollow sphere of the protein (Figure 12).
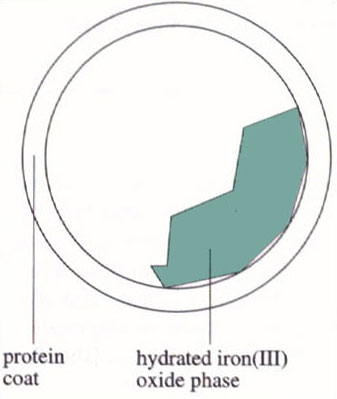
Therefore, ferritin stores iron as crystalline, hydrated iron(III) oxide within its structure
Activity 16
How will this affect the availability of the iron?
Answer
As the iron(III) oxide is very insoluble, it is unavailable to microbial iron-chelating ligands.
The inorganic iron(III) oxide core is also protected from chelators by the outer protein coat. Moreover, this is a very space-efficient method of storing iron; each ferritin protein macromolecule can store a maximum of 4 500 iron atoms.
Iron is delivered to ferritin (after having been transported by transferrin) where it migrates through the carboxylate-rich channels in the surface of the protein to the interior. The inner side of the protein sphere is also rich in carboxylate residues. It is thought that these carboxylate residues coordinate iron atoms, such that the iron atoms are held in a regular array. This regular array has the correct spacing of iron atoms to encourage the growth of crystals of iron(III) oxide, and in this way an iron(III) oxide phase grows within the ferritin core. The growth of iron(III) oxide in ferritin is an example of biomineralisation.
So it is somewhat ironic that the formation of highly insoluble iron(III) oxides and hydroxides, which causes such a problem in the availability of iron in the environment, is the method by which iron is stored in ferritin!
