Manufacture:
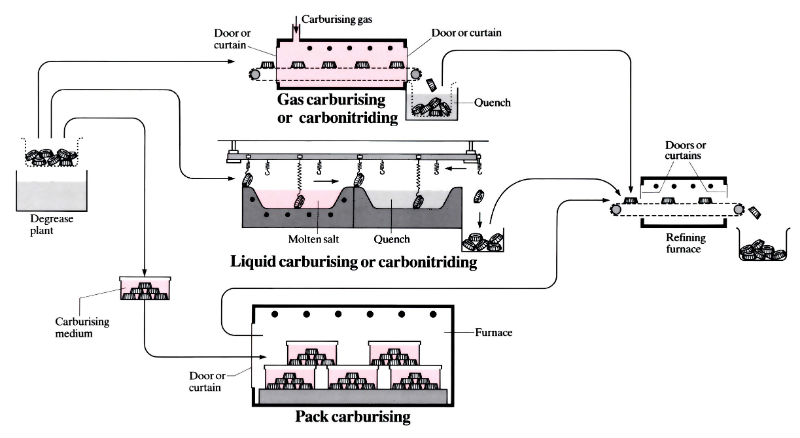
Gas carburising
- Continuous or batch furnaces which may be horizontal, vertical or rotary. More recently fluidised beds and reduced pressure furnaces have been used.
- The temperature is usually 925°C, although fluidised beds and reduced pressure furnaces operate up to 1050°C.
- The carburising atmosphere consists of natural gas or propane mixed with neutral “carrier gas”, usually a mixture of N2, CO, CO2, H2 and CH4.
- Carburising times vary with material, temperature and/or case thickness, but at 925°C can be as low as 2 h for a 1 mm case, or as high as 36 h for a 4 mm case.
- Quenching medium is usually oil but can be water, brine, caustic soda or polymer.
Liquid carburising (“cyaniding”)
- Carried out in a batch, automatic or semi-automatic salt baths heated by gas, oil or electricity. Brazing and carburising can sometimes be done simultaneously.
- Temperatures usually 845–955°C.
- Salt is usually a cyanide-chloride-carbonate mixture and is highly toxic. Cyanide salts also introduce a small amount of nitrogen into the surface of the component. Salt baths are available in which there is no cyanide, the carbon being in suspension in the salt.
- Times depend on the material, temperature and/or case depth, but are usually less than for gas or pack carburising, being in the range 2–10 h.
- Quenching as for gas carburising.
Solid (pack) carburising
- Component(s) placed in a sealed box, surrounded with the carburising medium and placed into a batch or continuous furnace. Boxes can be plain carbon steel for short runs or special heat- and corrosion-resistant alloys for long runs.
- Temperatures usually 850–950°C.
- Carburising medium is usually coke or charcoal mixed with a barium carbonate energiser, and the process is basically one of gas carburising since the CO produced dissociates into carbon dioxide and carbon, which diffuses into the surface.
- Times depend on material, temperature and case depth, see below.
Carbonitriding
- Almost any furnace which can be used for gas carburising can be adapted for carbonitriding.
- Temperatures usually 790–845°C.
- The atmosphere consists of a neutral “carrier gas” (similar to gas carburisation), an “enriching gas”, which is the source of carbon (natural gas or propane), and ammonia, which is the source of nitrogen.
- Times depend on material, gas composition temperature and/or case depth, but can be as low as 1 h, due to small case depths.
- Quenching as for gas carburising.
Variation of the depth of case with time and temperature:
| Time required | ||||
| Depth of case | 870°C | 900°C | 950°C | 960°C |
| 0.4 mm | 3.5 | 3 | 2.5 | 2 |
| 0.8 mm | 7 | 6 | 5 | 4 |
| 1.6 mm | 13 | 10 | 8 | 6 |
| 3.2 mm | 25 | 18 | 14 | 11.5 |
Materials:
Carburising
- The process relies on the transformation of austenite into martensite on quenching, so the carbon content of the component on the surface must be high enough (usually 0.8–1.0% C) to give a martensitic layer with sufficient hardness (700 VPN) to provide the required wear resistance.
- Since the core must have a degree of toughness, its carbon content should be a maximum of 0.4% and is usually less than 0.25%.
- Carried out on a wide range of plain carbon steels, alloy steels and cast irons.
- Incorrect heat treatment can lead to oxidation or decarburisation.
- Long heat treatment times at high temperatures can lead to grain growth of the core, necessitating core “refining” before quenching.
Carbonitriding
- Carried out on a similar range of steels as carburising, although carbon contents can be as high as 0.4–0.5% when a reasonably tough, through-hardened core is required with a hard surface, such as for gears and shafts.
- The composition of the case depends on the material, heat treatment atmosphere, time and temperature. The higher the temperature, the less effect the ammonia has, and the less nitrogen in the steel. At lower temperatures, iron-carbon-nitrogen compounds are formed at the surface.
- Gives a lower critical cooling rate than carburising, enabling the use of lower-carbon steels. Carbonitrided surfaces also resist softening on tempering better than carburised layers.
Design
- The shape and size of components are limited mainly by furnace size and capacity, although pack and liquid carburising may not be suitable for hardening inside small deep holes and recesses.
- Selected areas of a component can be hardened by “stopping off” the remaining areas using copper plating, copper or ceramic paints, copper or clay plugs.
- Distortion occurs in all processes; the lower the temperature, the less the distortion.
- Case depths and tolerances vary widely according to process, material, time and temperature. Typical case depths are:
- Gas carburising 0.5–5 mm
- Liquid carburising 0.075–3 mm
- Pack carburising 0.5–5 mm
- Carbonitriding <0.75 mm
- Tolerances vary from ±0.01 to ±1 mm but are poor for pack carburising.
See Also: Physical vapour deposition (PVD), Chemical vapour deposition (CVD) and Plasma nitriding/carburising.
This article is a part of Manupedia, a collection of information about some of the processes used to convert materials into useful objects.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews