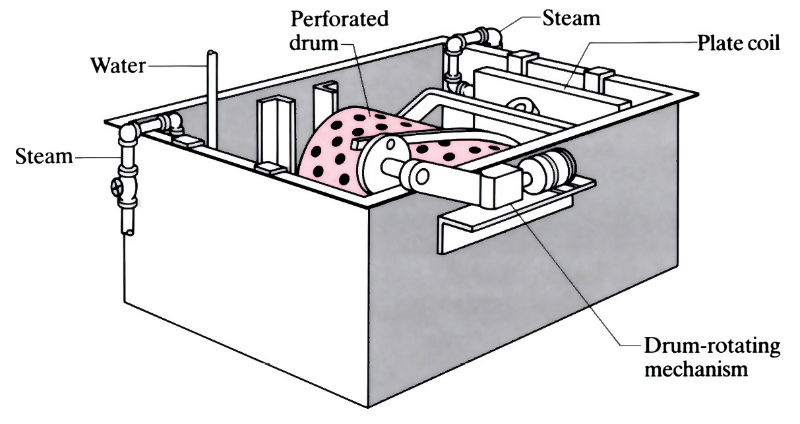
Typical immersion phosphating tank for batch coating of small parts. Drum into which parts are loaded is shown in immersion position.
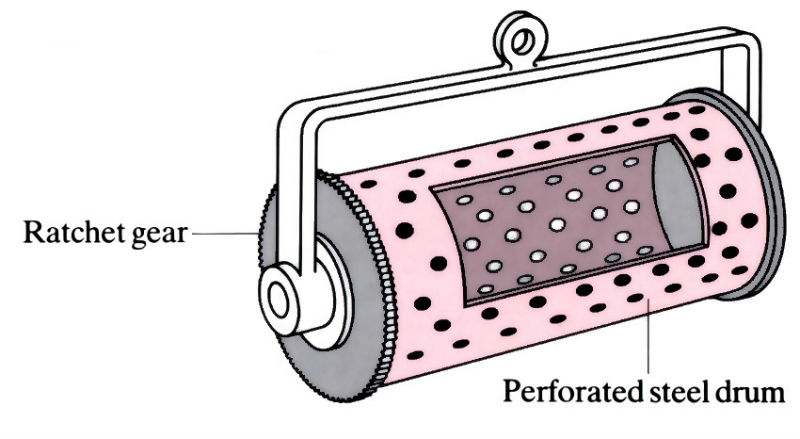
Typical drum used in batch phosphate coating of small parts.
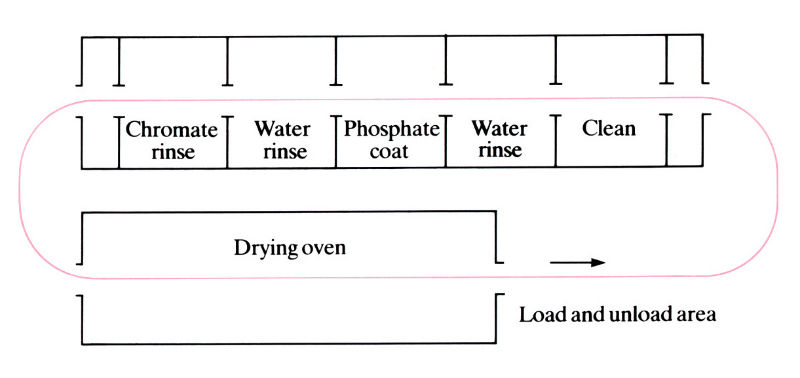
Plan of a typical continuous conveyor spray line for phosphating.
"Phosphate coating" is usually applied to the treatment of ferrous components with dilute phosphoric acid and other chemicals, whereby the surface of the metal is converted to a mildly protective layer of insoluble crystalline phosphate (2.5–50 µm thick). There are three main types: zinc, iron and manganese phosphates. The chemicals react with the metal and neutralise a thin film of the solution at the interface. In the neutralised film the solubility of metal phosphates is reduced, and they precipitate as crystals which are attracted to the surface of the metal electrostatically. Hydrogen embrittlement does not occur, unlike with electroplating. Accelerators, such as nitrates, chlorate or peroxide, speed up the coating reaction.
Manufacture:
- The method of applying phosphate coatings is usually determined by the size and shape of the article to be plated.
- Small items, such as nuts, bolts, screws and small stampings, are coating in tumbling barrels immersed in the phosphating bath. The drums are rotated at 4 rev min-1.
- Low-volume medium-sized parts are manually immersed in the tank using baskets.
- Large fabricated articles such as refrigerator bodies are spray-coated with the solution while they are on conveyors (300–1000 parts h-1). Only zinc and iron phosphate coatings can be sprayed. Usually used for high-volume coating of large parts. It is cheaper and quicker than the immersion technique:
- immersion 2.2 g m-2 takes 1 min
- spray 2.2 g m-2 takes 2 min
- Bath temperatures range from 45° to 105°C.
- Largest capacity available in the UK can treat up to 8 tons of 8 mm thick steel measuring 14 x 2 m for shipbuilding in a tank holding 122,000 litres of zinc phosphating solution.
- Chromate conversion coatings are produced by immersion into a tank of aqueous solution of chromic acid or chromium salts, such as sodium or potassium chromate or dichromate.
- Bonded fluorocarbon polymer coatings (PTFE) can be applied after phosphating by a dip, spin and bake (200°C) procedure (XYLAN XL™).
Materials:
- Phosphate coatings are normally applied to iron and steel components as a base for painting or as a lubricant for metal deformation processes such as wire drawing.
- Zinc phosphates vary in colour depending on the carbon content of the steel (the higher the carbon content, the darker the coating). Used as lubricant.
- Iron phosphates are chiefly used as a base for subsequent painting, and have excellent adhesion.
- Manganese phosphates are used on ferrous parts such as internal combustion (IC) engine components, for breaking in and to prevent galling. They are usually brown-black or black.
- Copper additions to low carbon steel prevent successful phosphating (>0.3% Cu).
- Low alloy, high strength steel can be phosphated provided the nickel and chromium contents do not exceed 1%. Stainless steels and electrical steels 1.2–4.5% Si cannot be phosphated.
- Cast irons can be phosphated (if surface is clean).
- Aluminium, magnesium, zinc and cadmium can be chromate conversion coated: 100,000 tons per year of aluminium is chromate treated as an intermediate coating and on painted or lacquered strip.
- Spray-applied PTFE coatings (XYLAN XL™) have good marine corrosion protection and low coefficients of friction.
Design:
- Phosphate coatings are increasingly being used as a base before final painting, e.g. car bodies (1 g m-2).
- Coating thicknesses range from 2.5 to 50 µm.
- Spray coatings range from 1 to 10 gm-2. Immersion coatings from 1.5 to 45 g m-2 (figures for zinc phosphate).
- No treatments are required to eliminate hydrogen embrittlement problems, as in electroplating.
- Other applications also include:
- a base for oil or other rust-preventive material
- lubricity and resistance to wear, galling or scoring of parts moving in contact, e.g. parts in IC engines
- a surface that facilitates cold forming of components
- a base for adhesive in plastic-metal laminations
- Bonded fluorocarbon polymer coatings (PTFE) have been applied to cooking utensils and hacksaw blades to reduce friction.
See Also: Electroless plating, Electroplating, Anodising and Chemical vapour deposition (CVD)
This article is a part of Manupedia, a collection of information about some of the processes used to convert materials into useful objects.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews