7 Oil and anthropogenic global warming
Since the mid-19th century, human industrial and agricultural activity has caused a change in the concentrations of gases in the Earth's atmosphere. In particular, there has been a dramatic increase in the concentration of atmospheric carbon dioxide (Box 1), caused primarily by the combustion of fossil fuels.
Box 1 Carbon dioxide and chemical formulas
Carbon dioxide is a colourless, non-toxic gas which occurs naturally in the atmosphere. It is produced whenever compounds containing carbon are burnt in air, and is also produced as a result of respiration - the process by which organisms (including humans) convert their food into energy. Carbon dioxide has the chemical formula CO2. This means that carbon dioxide gas is made of tiny units each comprising one atom of carbon and two atoms of oxygen. These units are called molecules. (The subscript '1' is assumed if no other number is written.) It's important to note the way the chemical formula is written: capital C (for carbon), capital O (for oxygen) followed by a subscript 2, i.e. it is CO2 not CO2 or co2.
Figure 12 shows the emissions of carbon dioxide resulting from combustion of fossil fuels (and cement production) over the last 200 years.
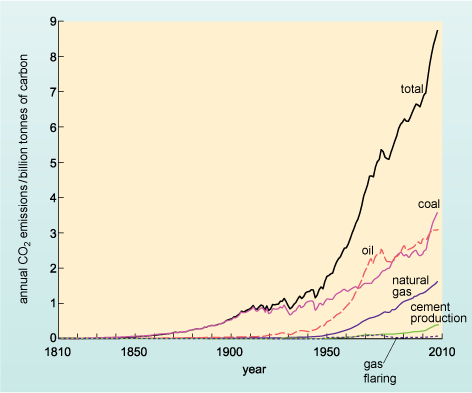
Examine Figure 12. Until the 1950s, which fuel contributed most to anthropogenic carbon dioxide emissions?
Coal.
Approximately when were the emissions from this fuel first overtaken by those of oil?
In about 1970.
At present the combustion of coal and oil are responsible for broadly similar levels of CO2 emissions, whilst emissions from the combustion of natural gas are approximately half those of either coal or oil.
Natural gas consists primarily of methane, a substance made up of carbon and hydrogen. Given that each molecule of methane contains one atom of carbon and four atoms of hydrogen (symbol H), what is the correct way to write the chemical formula of methane?
CH4 - capital C (carbon), capital H (hydrogen), with subscript 4. If you wrote H4C you are not, strictly speaking, wrong but chemists always tend to put carbon at the beginning of a chemical formula.
In order to more fully describe the combustion of coal, natural gas and oil, it is necessary to use chemical equations, as described in Box 2. If you have studied chemistry recently, the concepts may be relatively familiar and you may be able to read through this box quickly, or skip it completely.
Box 2 Introducing chemical equations
When chemists want to describe a chemical reaction in which one set of substances (the reactants) are transformed into another (the products), they usually do so by means of a chemical equation. The reactants are shown on the left with an arrow leading to the products on the right:
Coal is made up mostly of carbon atoms (C). Oxygen in the air is made up of pairs of oxygen atoms, written as O2. When coal burns, the carbon and oxygen atoms combine to make carbon dioxide. The ash left after a coal fire is largely the part of the coal that was not carbon and so did not burn away to carbon dioxide.
The combustion reaction between carbon and oxygen would be written like this:
Notice that there must be the same amount of carbon and the same amount of oxygen (measured here by the number of atoms) on each side, since you cannot create more carbon or oxygen, or lose any, in a chemical process. This applies to all chemical equations like this. They must be 'balanced' with the same number of atoms on each side. It is also useful to indicate whether the substances taking part in the reaction are solids, liquids or gases, so the letter s, l or g is placed in brackets after each substance. So, since the coal is a solid, and the oxygen and carbon dioxide are gases, the complete version of reaction 1.1 would be:
Now consider what happens when natural gas, methane CH4, is burnt in a similar way. This time the products include water, whose chemical formula is H2O.
Try writing the equation in which methane burns in oxygen to produce carbon dioxide and water, in the form of steam. Remember to include the symbols that show whether the substances taking part are solids, liquids or gases.
The equation is:
CH4(g) + O2(g) → CO2(g) + H2O(g)
The numbers of atoms on each side are not the same; there are three oxygen atoms on the right-hand side, but only two on the left. Since the oxygen atoms are in pairs on the left, there needs to be an even number on the right too. That can be done if the reaction produces two molecules of water. So the next stage is to write:
CH4(g) + O2(g) → CO2(g) + 2H2O(g)
Now, counting the carbon atoms shows that there is one on each side, and there are four hydrogen atoms on each side (four in the methane on the left, and two water molecules, each with two hydrogen atoms, so that makes four on the right too). However, there are only two oxygen atoms on the left and four on the right (two in CO2 and one in each of the two water molecules). But if you have two molecules of oxygen on the left, then that gives four oxygen atoms on both sides too. The final equation for the combustion of methane can be written:
An equation like this, with equal numbers of atoms on both sides, is said to be balanced, and often a fully balanced equation will be written with an equals sign (=) to replace the arrow (→). So the equation can also be written as:
A chemical equation written with an 'equals' sign emphasises that it is balanced, and this form is often used in Open University texts.
Question 4
Propane is a gas derived from the processing of crude oil. It is used as a fuel for heating and cooking. Propane consists of molecules containing three atoms of carbon and eight atoms of hydrogen. Give the chemical formula of propane and write an unbalanced chemical equation to show its combustion.
Answer
The chemical formula of propane is C3H8 - it is very important that the letters C and H are capitals, and that the numbers are subscripts.
An unbalanced chemical equation for the combustion of propane is:
Note that the unbalanced equation uses an arrow. The full balanced equation, which you were not asked to give, can be written:
Once again the full balanced equation can be written using an 'equals' sign (=) rather than an arrow (→).
The fossil fuels burned in the 200 years since the Industrial Revolution were formed underground over many millions of years. The sudden return of ancient carbon to the atmosphere upsets the outer Earth's energy balance, and is responsible for anthropogenic global warming. This is an issue of much concern among scientists, environmentalists and politicians.
The science behind global warming will not be discussed in any detail in this course; but the following text will discuss briefly why gases like carbon dioxide affect the global temperatures. A number of gases like CO2 absorb some of the Sun's energy which might otherwise be reflected back into space, resulting in an overall warming of the atmosphere. Gases that can act in this way are called greenhouses gases. The two major greenhouse gases are water vapour (H2O) and carbon dioxide (CO2), although methane (CH4) is also an important greenhouse gas. Without the warming effect of these naturally occurring gases, the temperature at the Earth's surface would be closer to −20 °C than the current mean value of 15 °C. Increases in carbon dioxide and other greenhouse gas concentrations will inevitably lead to increases in global mean surface temperatures.
The increasing concentration of CO2 in the atmosphere has already resulted in small but measurable increases in global temperatures. In fact, the 20th century saw an increase in the annual mean surface temperature of about 0.7 °C. Predicting future global warming is beset with uncertainties, but our best estimates are that if there is no change in the current 'blend' of energy sources (known as the 'business-as-usual' scenario), the global mean surface temperature will rise by between 1.1 °C and 6.4 °C over the next 100 years or so.
Increased surface temperatures are likely to have a variety of effects. One major effect would be on sea levels: increases in global temperature are likely to be accompanied by a rise in global sea level, because of thermal expansion of the oceans and melting of land-based ice. A 'business-as-usual' scenario suggests a sea-level rise of between 0.2 m and 0.65 m by the end of the 21st century. Low-lying island states are particularly threatened by rising sea levels; but other effects could include greater incidence of storm-surge flooding, higher coastal erosion, and loss of property and coastal habitats. Rising sea levels could have devastating social and economic consequences.
Other effects of anthropogenic global warming include changes to the timings of the seasons, with effects on agriculture, changing distribution of diseases and damage to ecosystems.
To stabilise carbon dioxide levels would require an immediate reduction of emissions from human activities. Yet even if fossil fuel burning stopped immediately, the temperature rise would continue because of the time lags in atmospheric systems.
The combustion of oil is a major contributor to carbon dioxide emissions - contributing just over one-third of all anthropogenic emissions (Figure 12), a proportion similar to the emissions produced from coal, which is primarily burnt to generate electricity.
Clearly if we are to reduce emissions of carbon dioxide, reducing our reliance on oil will not by itself be enough - we definitely need to do something to replace our coal-fired power stations. Nonetheless, developing sustainable, efficient alternatives to oil-based fuels could make a major contribution to efforts to reduce greenhouse gas emissions.
