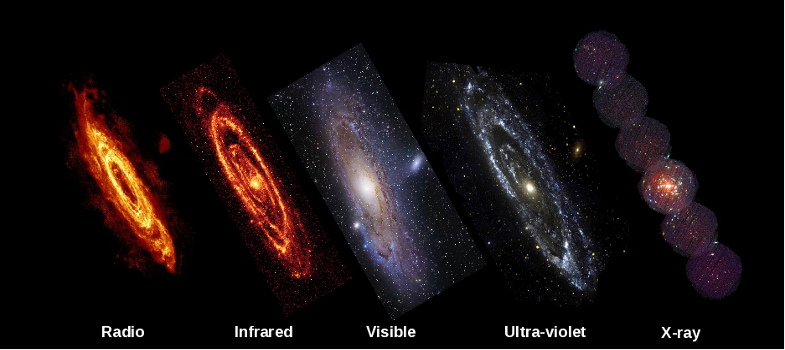
Section 5.1.1: What do the colours in a rainbow tell us?
rainbows
When a beam of white light, such as that from the Sun, is passed through a glass prism, it is broken up, or dispersed, into a band of colours that is called the visible spectrum. This is also familiar as the rainbow that we see when raindrops disperse light in the same way. The spectrum of sunlight contains visible light of all colours and therefore forms a continuous spectrum.

(credit: Eric Rolph)
The reason that some things appear to be coloured is that atoms and molecules absorb and emit different colours of light. So, the petal of a rose, for instance, appears red when sunlight shines on it because the petal absorbs all the visible colours except the red light. Some of the red light passes through the petal, and some is reflected from its surface.
It turns out that every type of atom shows a preference for absorbing or emitting light of certain specific colours. So each atom is associated with a characteristic pattern of colours – a sort of technicolour fingerprint. Just as every human being has their own set of characteristic fingerprints, every type of atom has its own pattern of colours of light that it can absorb or emit.
One example is the yellow glow produced by ‘sodium’ street lights. This occurs when an electric current passes through vaporised sodium, hence the yellow glow is the fingerprint of that element. Similarly, the bright orange–red light of a neon sign is the fingerprint of the element neon. Such associations are even more apparent when a prism is used to disperse the light from a source of known chemical composition. In this case, spectra consisting of emission lines are seen.
Exactly the same association may be seen by observing how white light is absorbed by atoms. This is done by passing a beam of white light through some vapour and examining the spectrum of the emerging beam to see if any colours are missing. The resulting absorption spectrum exhibits dark absorption lines, marking the absence of exactly the same colours that were seen in the emission spectrum. Sodium and neon atoms are by no means unique in having a characteristic pattern of spectral lines. In fact, every kind of atom has an associated, characteristic ‘spectral fingerprint’.
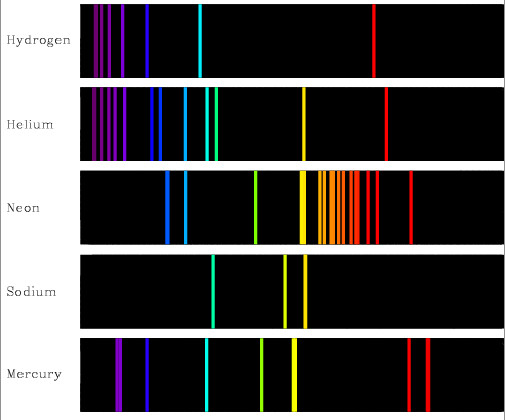
Emission spectra of some common elements (credit: NMSU, N. Vogt)